| Summary |
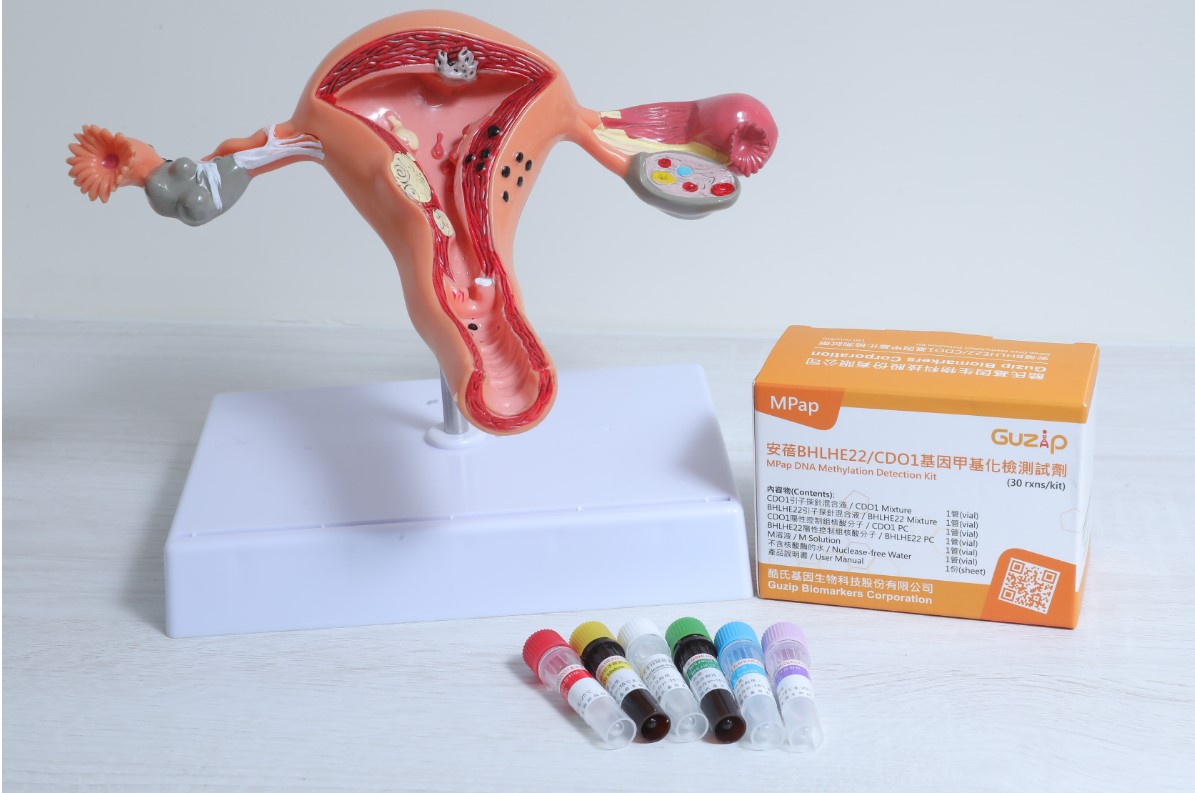
The MPap DNA Methylation Test is an in vitro diagnostic reagent for endometrial cancer detection. For women atover 40 years of age with abnormal uterine bleeding, the methylation status of BHLHE22CDO1 genes from conventional Pap smear, can be used as an auxiliary diagnosis of endometrial cancer. The test result provides an important triage for further invasive endometrial biopsy, which will substantially reduce the need of invasive proceduresincrease the feasibility of endometrial cancer screening in high risk populations. This is a ground breaking test.
|
| Scientific Breakthrough |
There is no molecular testing for endometrial cancer detection so far. The MPap DNA Test, based on DNA methylation from Pap smear, is the first in class product. From a clinical trial of 5 medical centers in Taiwan in 2018-2020, MPap, with sensitivity, specificity, PPVNPV of 92.5, 73.8, 40.2,98.1, respectively, all outperformed transvaginal ultrasound. This is the first multicenter study to validate the performance of methylation biomarker-based testing for EC detectionperform a head-to-head comparison of such testing with transvaginal ultrasound.
|