| Technical Name | Dual-function non-platinum electrocatalyst for hydrogenoxygen production via water splitting | ||
|---|---|---|---|
| Project Operator | National Taiwan University | ||
| Project Host | 謝宗霖 | ||
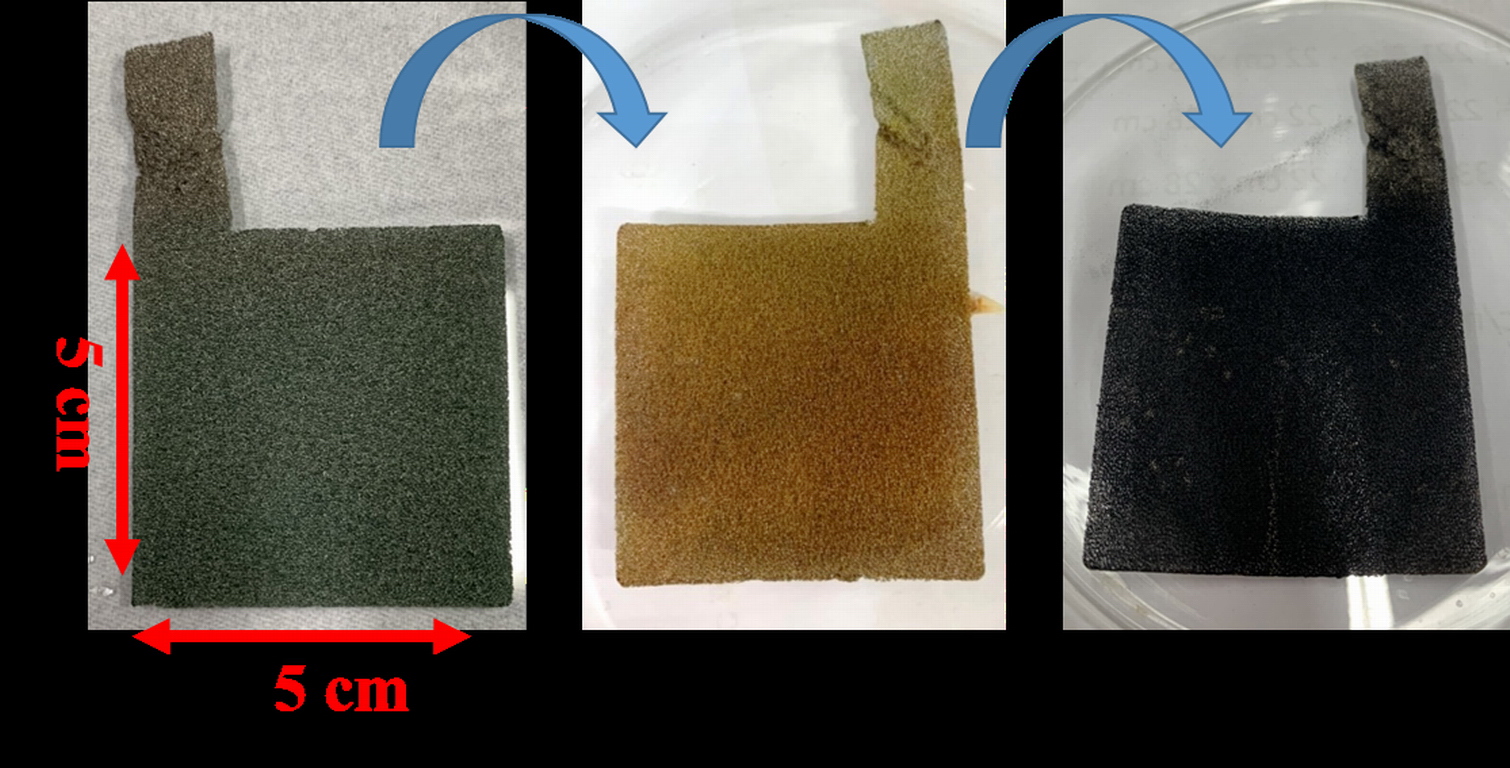
| Summary | Electrochemical water splitting is widely regarded as a feasible industrial hydrogen production technology that can efficiently convert water into hydrogen gas. This technology utilizes an in-situ growth method to grow Ni/Co bimetallic MOFs on Ni foam substratesthen undergo phosphorization treatment to prepare efficient, long-life,non-precious metal (non-Pt) dual-function (i.e., applicable to both hydrogenoxygen evolution reactions) electrocatalysts for water splitting. |
||
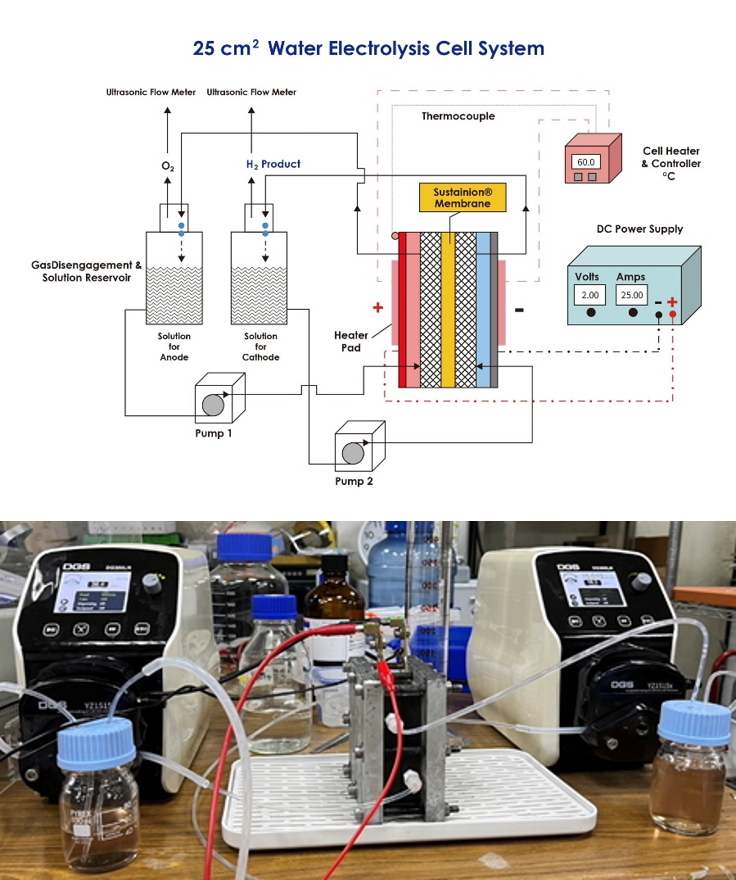
| Scientific Breakthrough | The phosphide NiCo/NF catalyst in this technology can be operated at a low driving voltage. Under alkaline conditions at a current density of 400 mA/cm2a duration of 72 h, the voltage required for hydrogen evolution is 2.5 V, with a hydrogen production rate of 75.9 mL/min (electrode area = 25 cm2). The Faradaic efficiency is close to 100. The energy consumption for hydrogen production per cubic meter is 5.49 kWh/Nm3, which is comparable to commercial precious metal electrocatalysts. |
||
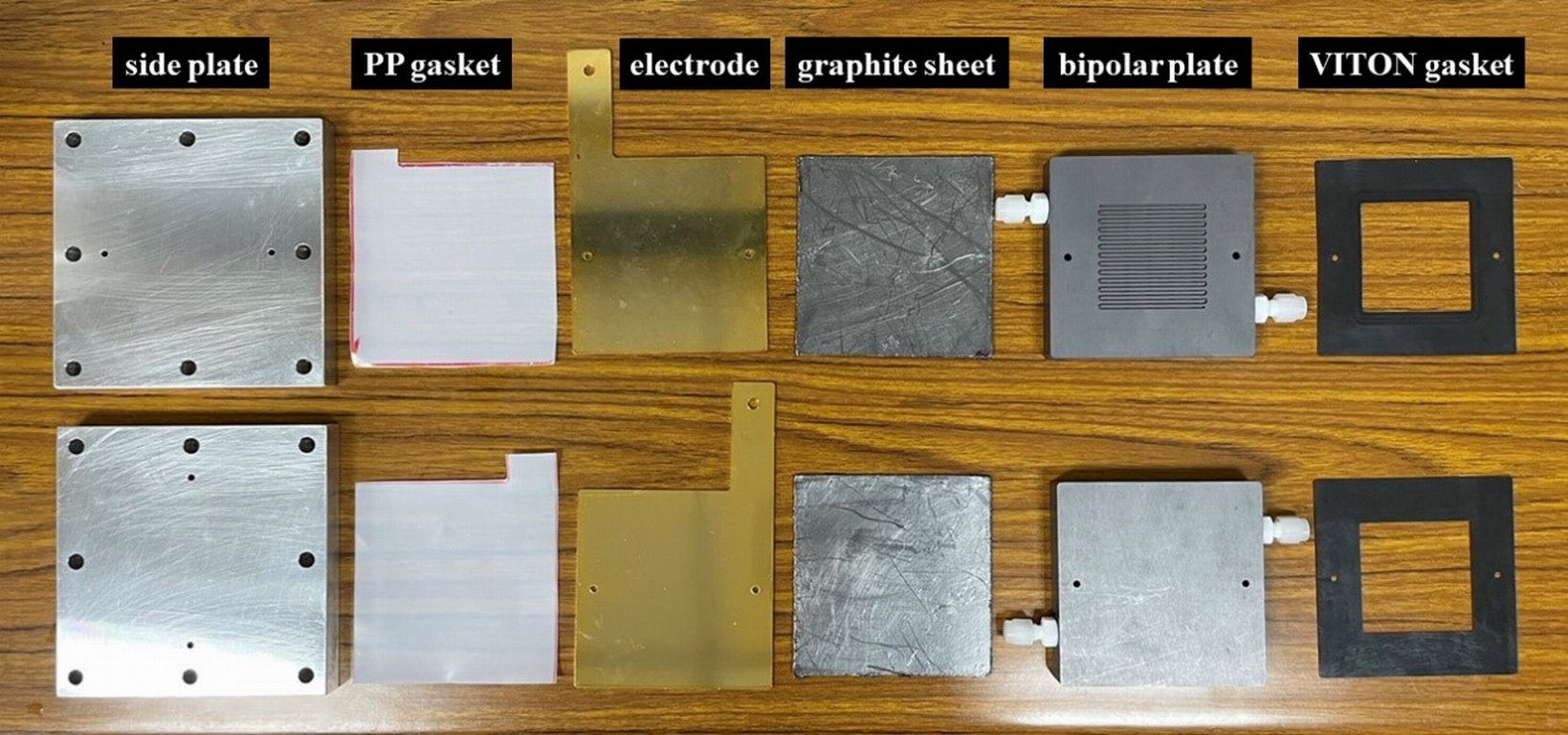
| Industrial Applicability | This technology applies the large-area phosphide NiCo/NF electrocatalyst to an alkaline AEM water electrolyzer to assess its feasibility in industrial operations. This dual-function non-Pt electrocatalyst is a scalablerelatively low-cost material technology that can be applied in various hydrogen productionstorage fields, such as hydrogen filling stations, long-distance transportation systems,hybrid energy storage systems (HESS) integrated with other green energy technologies. |
||
| Keyword | Dual-function electrochemical catalysts Non-Pt electrocatalysts Electrolytic hydrogen production Transition metal phosphides (TMP) Metal-organic frameworks (MOF) In situ growth Hydrogen evolution reaction (HER) Oxygen evolution reaction (OER) High current density Hydrogen production rate | ||
- Contact
- Tzong-Lin Jay Shieh
- jayshieh@ntu.edu.tw
other people also saw