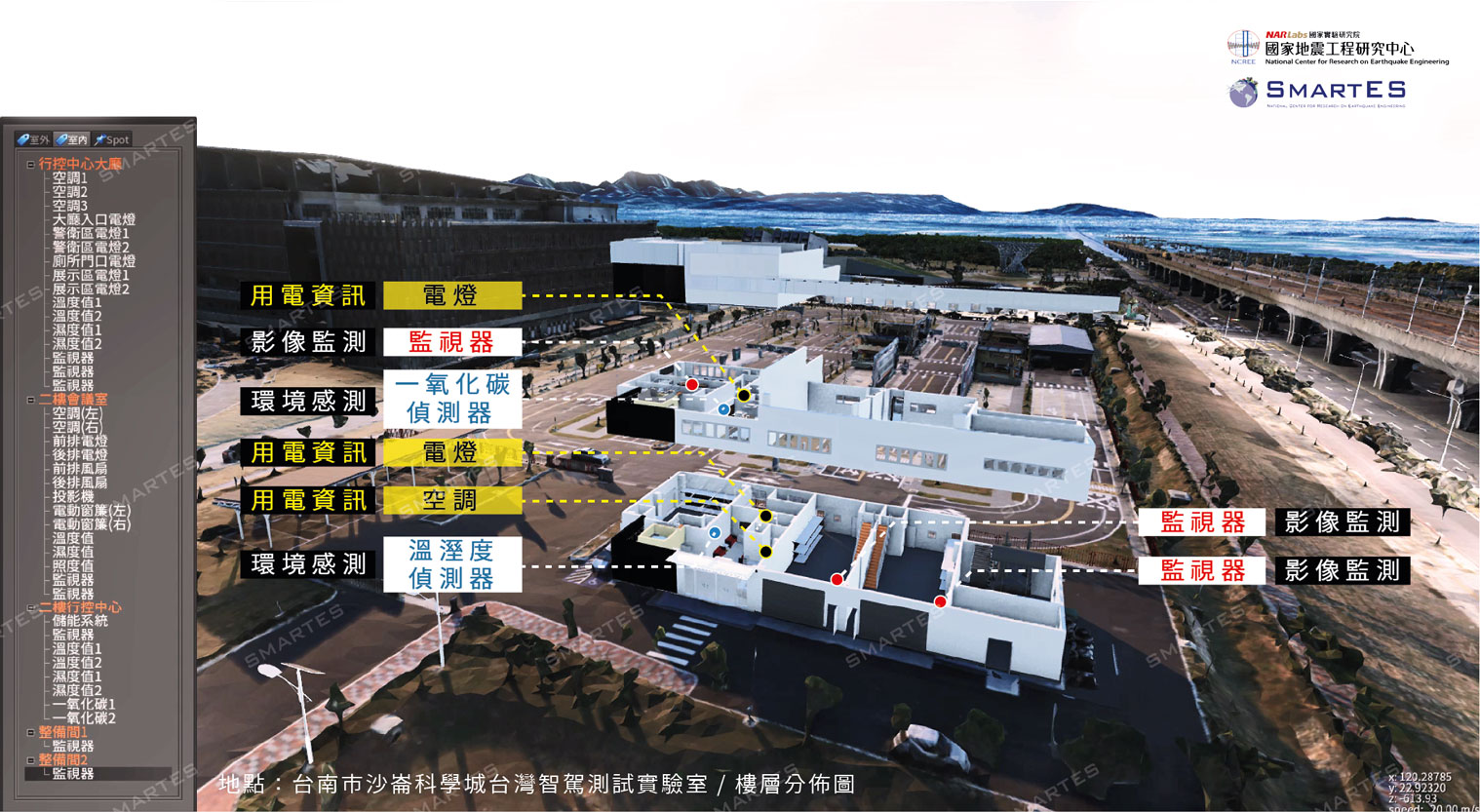
| 技術名稱 | Erafilm 速效生物膜清除組合 | ||
|---|---|---|---|
| 計畫單位 | 國立臺灣大學 | ||
| 計畫主持人 | 邱浩傑 | ||
| 技術簡介 | 細菌形成的生物膜是導致導尿管相關性感染(CAUTIs)的主因。目前,臨床上缺乏安全且有效的生物膜清除策略。我們的ERAfilm可在10分鐘內清除CAUTI病人所拔除之導尿管上近95的生物膜,對人類膀胱細胞無毒性,且不誘導細菌抗藥性,有望成為預防CAUTIs的重要解決方案。 |
||
| 科學突破性 | 生物膜為細菌的物理性屏障,可阻礙免疫系統和抗生素的攻擊。ERAfilm由兩種可穿透生物膜的小分子所組成,能快速清除導管上各式細菌的生物膜,且對人類細胞無毒性,不誘導細菌產生抗藥性。相比其他技術,ERAfilm在應用範圍、清除效率等方面具有明顯優勢,為一種具潛力用於預防CAUTIs的創新發明。 |
||
| 產業應用性 | ERAfilm是一種創新的生物膜清除技術,其主要應用目標是導尿管上的生物膜,以預防相關泌尿道感染症的發生。其非侵入式和簡易操作等特性使其在醫院、診所、長照機構和居家護理服務等領域具有廣泛的應用前景。隨著全球高齡化和需使用人工導管的患者增加的趨勢下,預期ERAfilm的需求與市場規模亦將逐年擴大。 |
||
| 關鍵字 | 生物膜 人工植入物 導尿管 尿路感染 膀胱灌洗 高齡化社會 居家照護 長期護理 預防醫學 創新技術 | ||
- 聯絡人
- 楊惠惠
- 電子信箱
- hui_970220@hotmail.com