| Technical Name | Zero Emissions: Low-cost Alkaline Seawater Electrolysis for Hydrogen Production: The DeviceTechnology | ||
|---|---|---|---|
| Project Operator | National Sun Yet-sen University | ||
| Project Host | 陳軍互 | ||
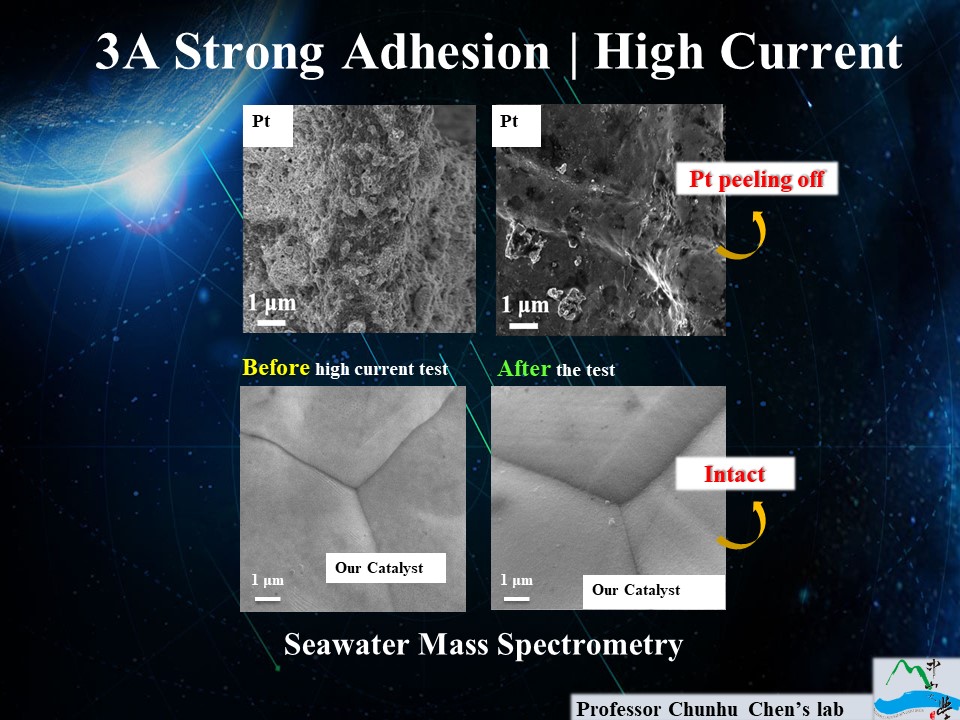
| Summary | We have developed a low-cost method to produce high-adhesion thin-film catalysts achieving H2 production efficiency of 71 (at 80 ºC) at a current density of 1000 mA cm-2,is stable for at least 5 hours at 0.4A/cm2 in the seawater. The excellent adhesion prevents catalyst removal in electrolyzer operation (using anion-exchange membrane, AEM), which can solve the difficulty of H2 production at high current densityhigh cost of the electrocatalysts. |
||
| Scientific Breakthrough | This technique (aqueous redox deposition, ARD) is driven by a spontaneous redox reaction. Electron transfer from the metal-containing oxidant to other metal precursors can produce a homogeneous, multimetal film at room temperature/ambient pressure without electricity. The multimetal-doped film catalyst operates beyond the limit of 0.5A as described in the literature, can achieve even under high current water splitting,is stable for at least 5 hours at 0.4A/cm2 in the seawater. |
||
| Industrial Applicability | The cost of our catalyst in water-splitting is 0.17 of noble metal catalysts,it can be large-scale produced by roll-to-roll process. The energy conversion efficiency can reach 71 (at 80°C),1000 mA cm-2 has passed the operation test for more than 720 hours. Our team's highest efficiency can reach 81, in this state, the cost of hydrogen can reach the same cost as fossil fuel,it can be used to drive electric buses, proving the extremely high industrial applicability. |
||
| Keyword | Green energy Energy storage Hydrogen Water splitting Large scale Energy efficiency Roll-to-roll Anion exchange membrane First row Transition metal Film Oxygen | ||
- Contact
- Chun-Hu Chen
- chunhu.chen@mail.nsysu.edu.tw
other people also saw