| Technical Name | An effectivesafe first-in-class new drug for abdominal pain in irritable bowel syndrome | ||
|---|---|---|---|
| Project Operator | National Taiwan University | ||
| Project Host | 忻凌偉 | ||
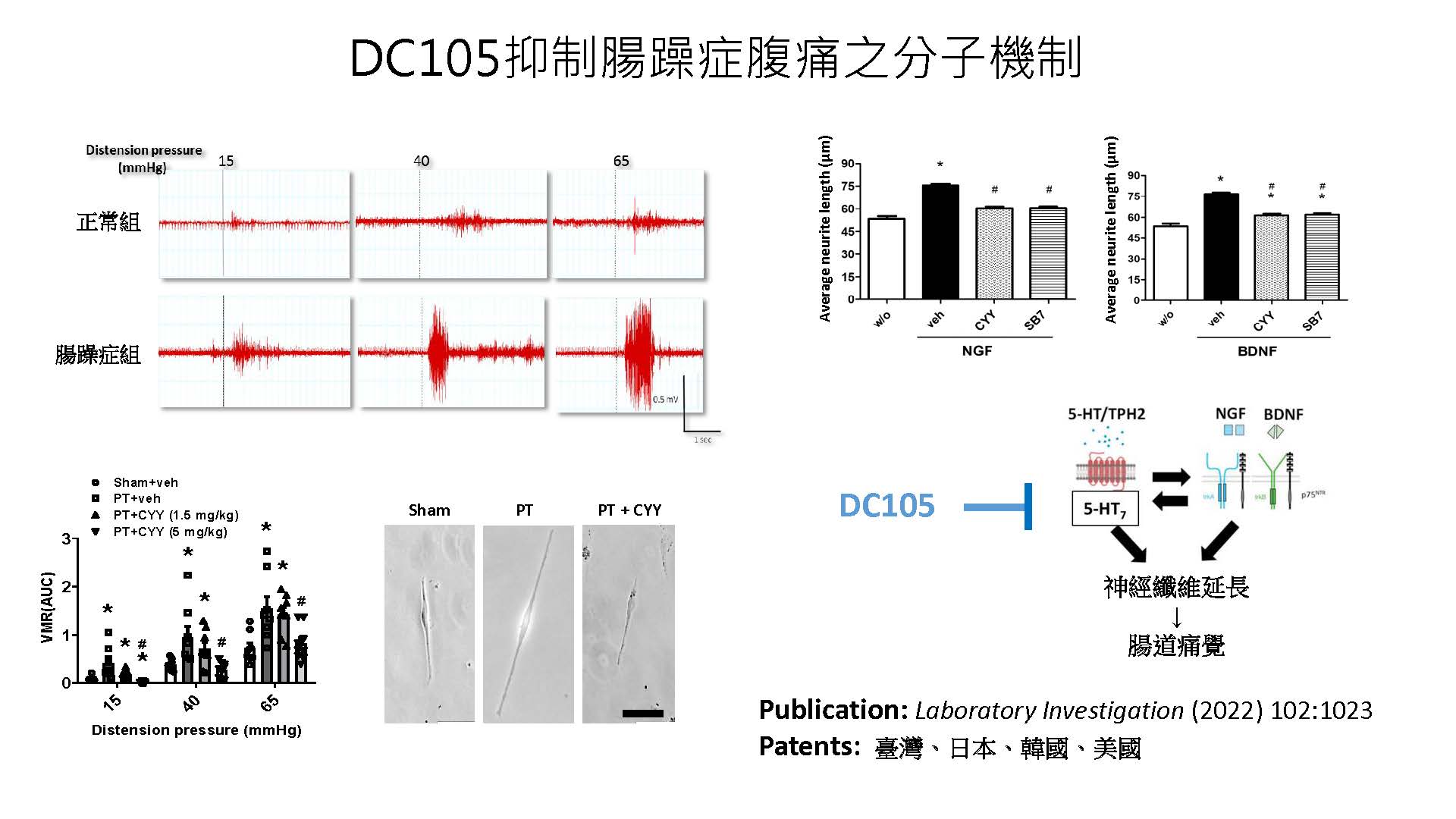
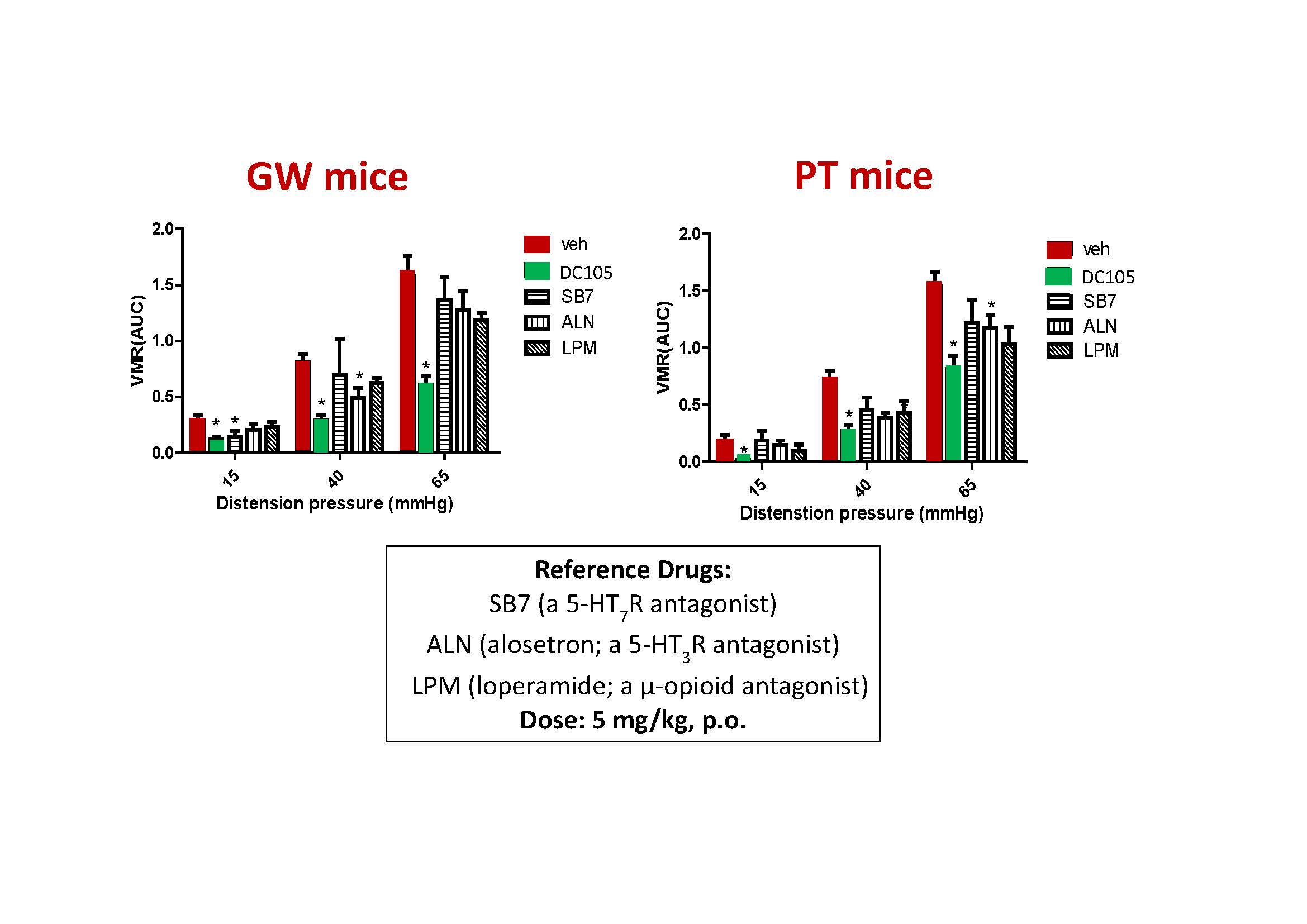
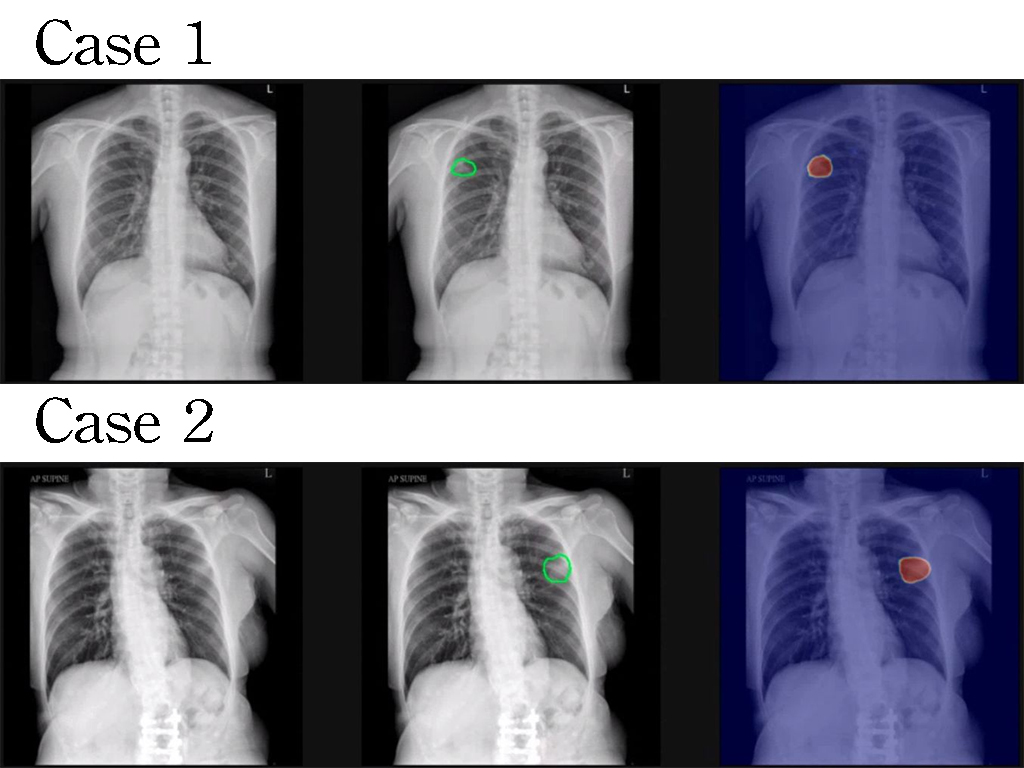
| Summary | IBS abdominal pain remains a highly unmet medical need. This invention selects the serotonin type-7 receptor as a new target for the treatment of IBS abdominal pain, establishes two IBS animal models to determine the ability of new drugs for inhibition of hyperalgesia. After drug designoptimization, DC105, a first-in-class medication for IBS abdominal pain, was selected with high target specificity, effective reduction of visceral hypersensitivity after oral administration,good safety. |
||
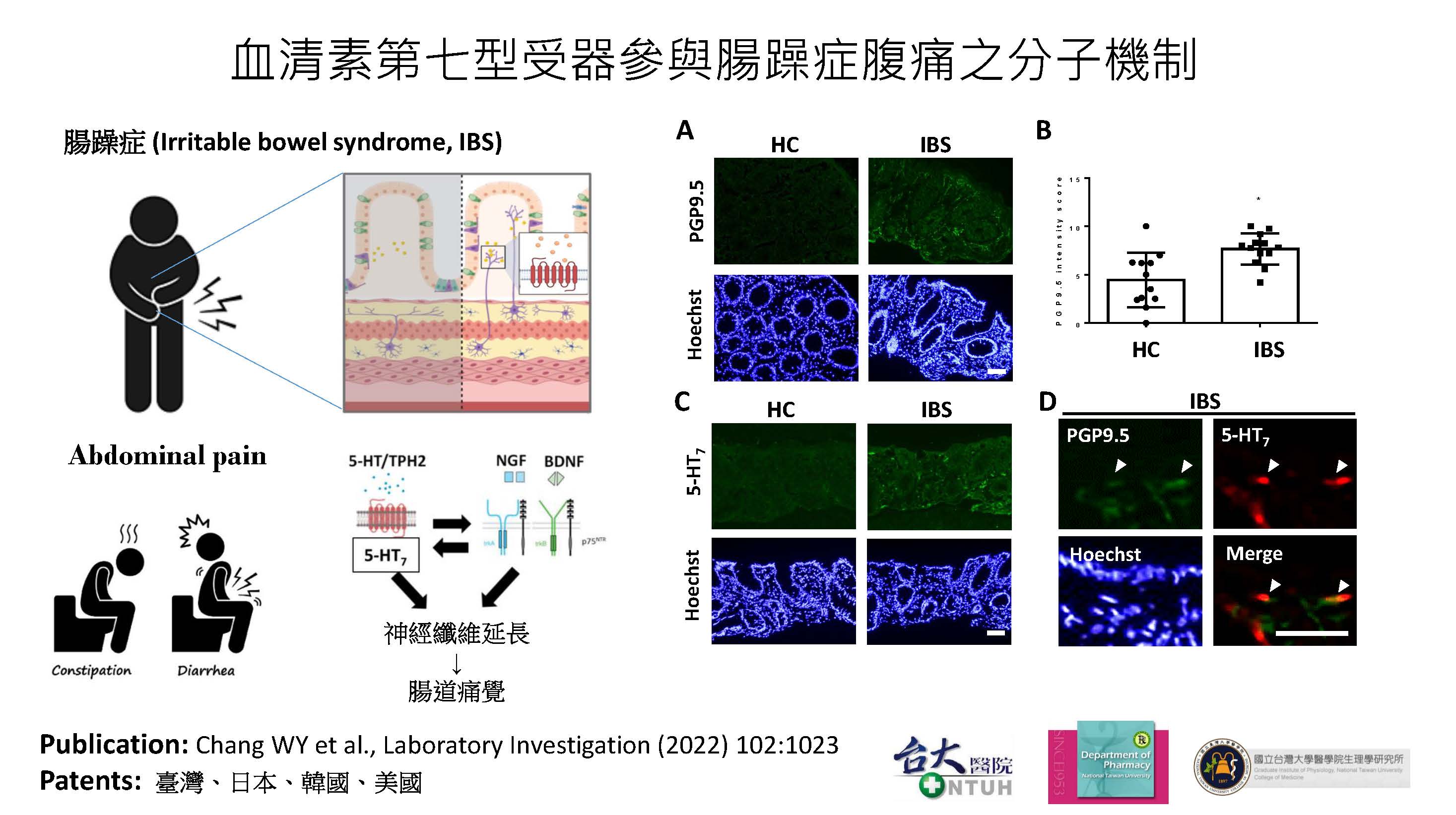
| Scientific Breakthrough | The abdominal pain in irritable bowel syndrome (IBS) is still an unmet need. This study aims to develop an effectivesafe treatment for IBS abdominal pain. The major scientific breakthroughs of this new technology are i) identification of serotonin type 7 receptor as a new target for the treatment of IBS abdominal pain ii) discovery of novel pharmacological mechanism for hyperalgesia in IBS iii) development of a first-in-class drug candidate DC105 for the treatment of IBS abdominal pain. |
||
| Industrial Applicability | Although Rifaximin has limited efficacy for IBS-D abdominal pain, its sale was $1.65 billion in 2021. DC-105 is a first-in-class small-molecule new drug for the treatment of IBS-D abdominal pain. The manufacturing process is economicalfeasible to scale up. DC-105 is protected by complete patent layoutits expected annual sales is $1~3 billion after it is launched. Evaluation of its potential for the treatment of constipation-predominant IBS patients will be the next stage. |
||
| Keyword | irritable bowel syndrome abdominal pain first-in-class preclinical study investigational new drug 5-HT7 receptor antagonist functional gastrointestinal disorder hyperalgesia new drug discovery | ||
- Contact
- Li-Te Chang
- litechang@ntu.edu.tw
other people also saw