| 技術名稱 | 安全有效的腸躁症腹痛首創型新藥 | ||
|---|---|---|---|
| 計畫單位 | 國立臺灣大學 | ||
| 計畫主持人 | 忻凌偉 | ||
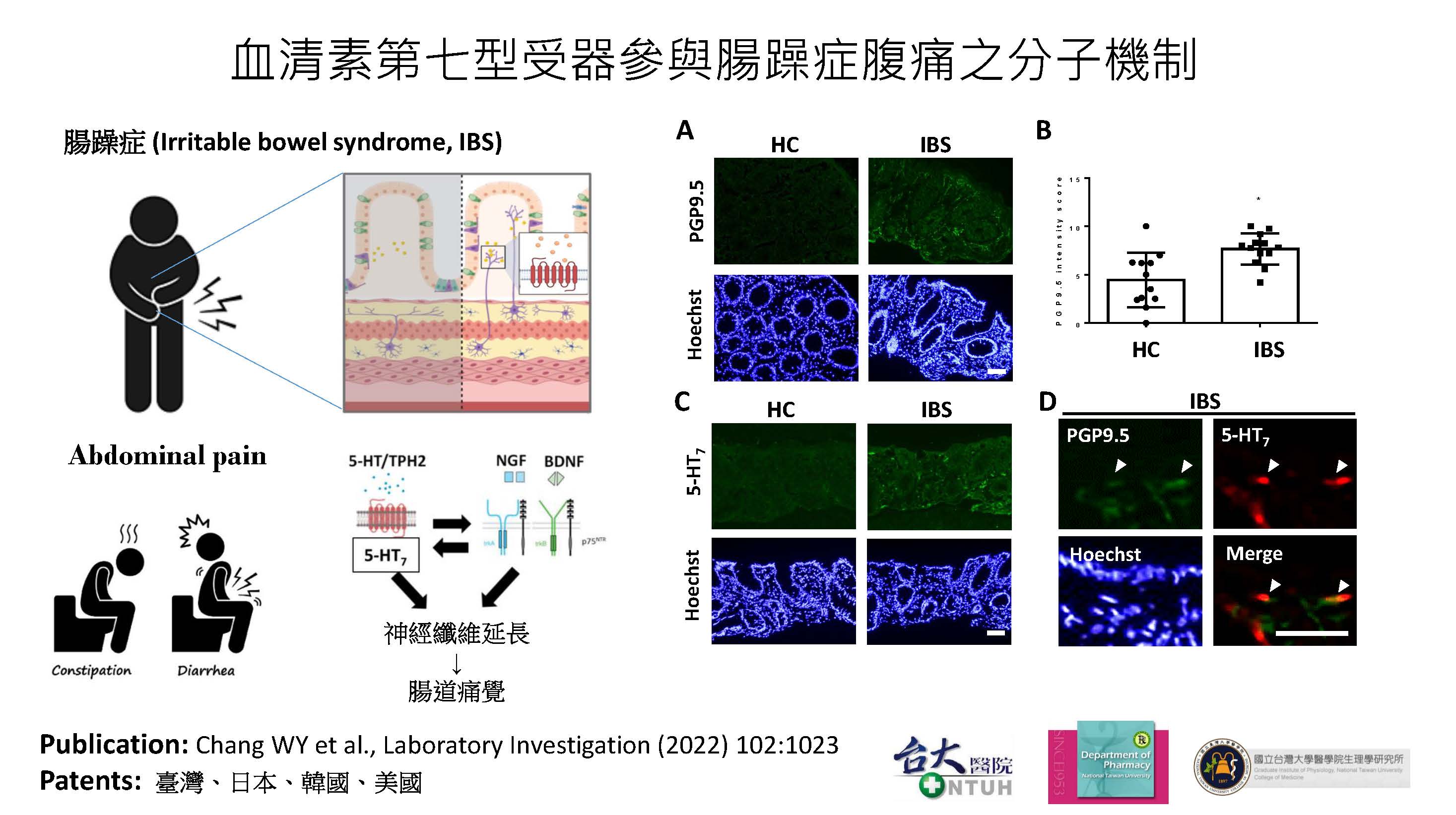
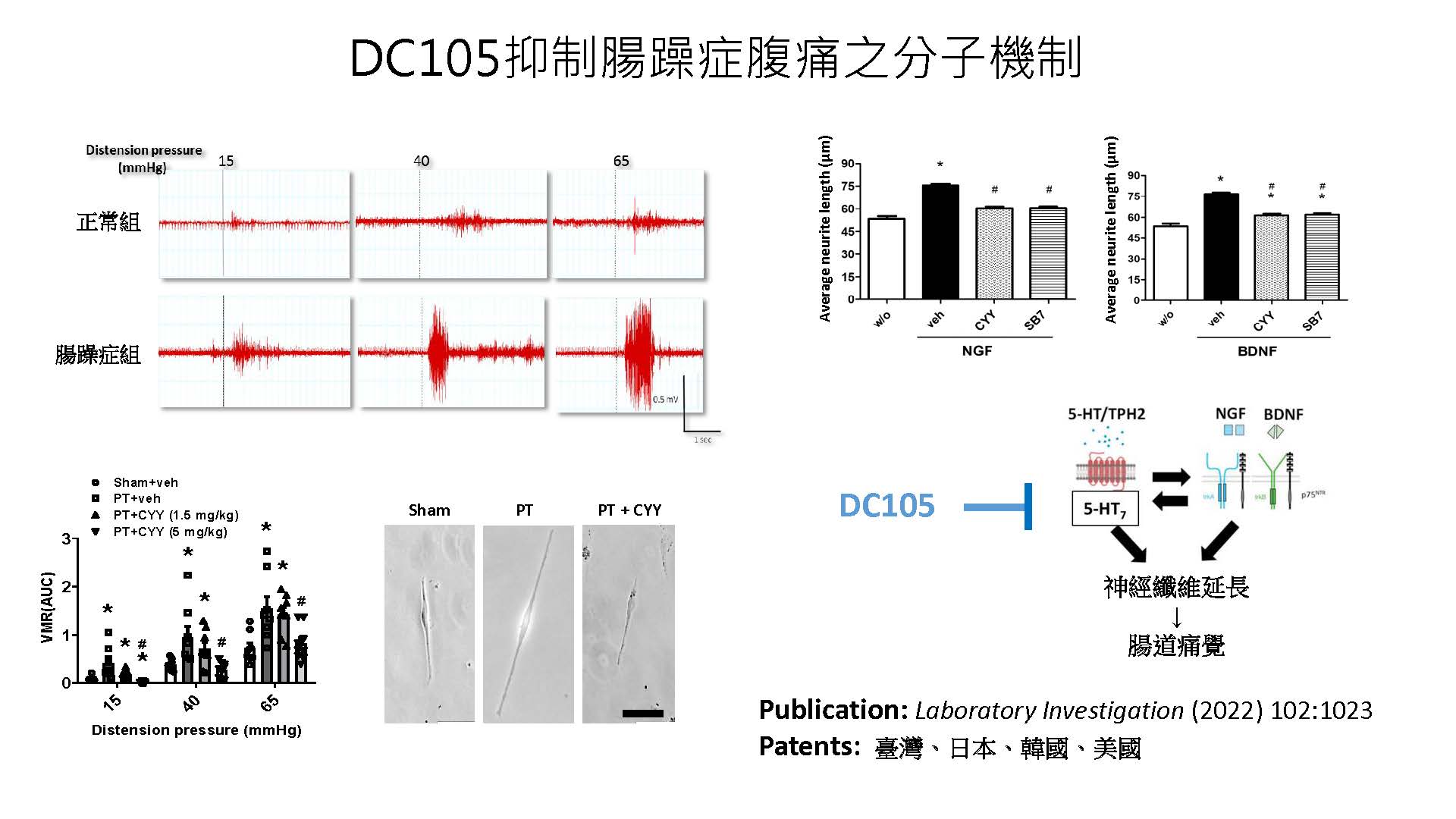
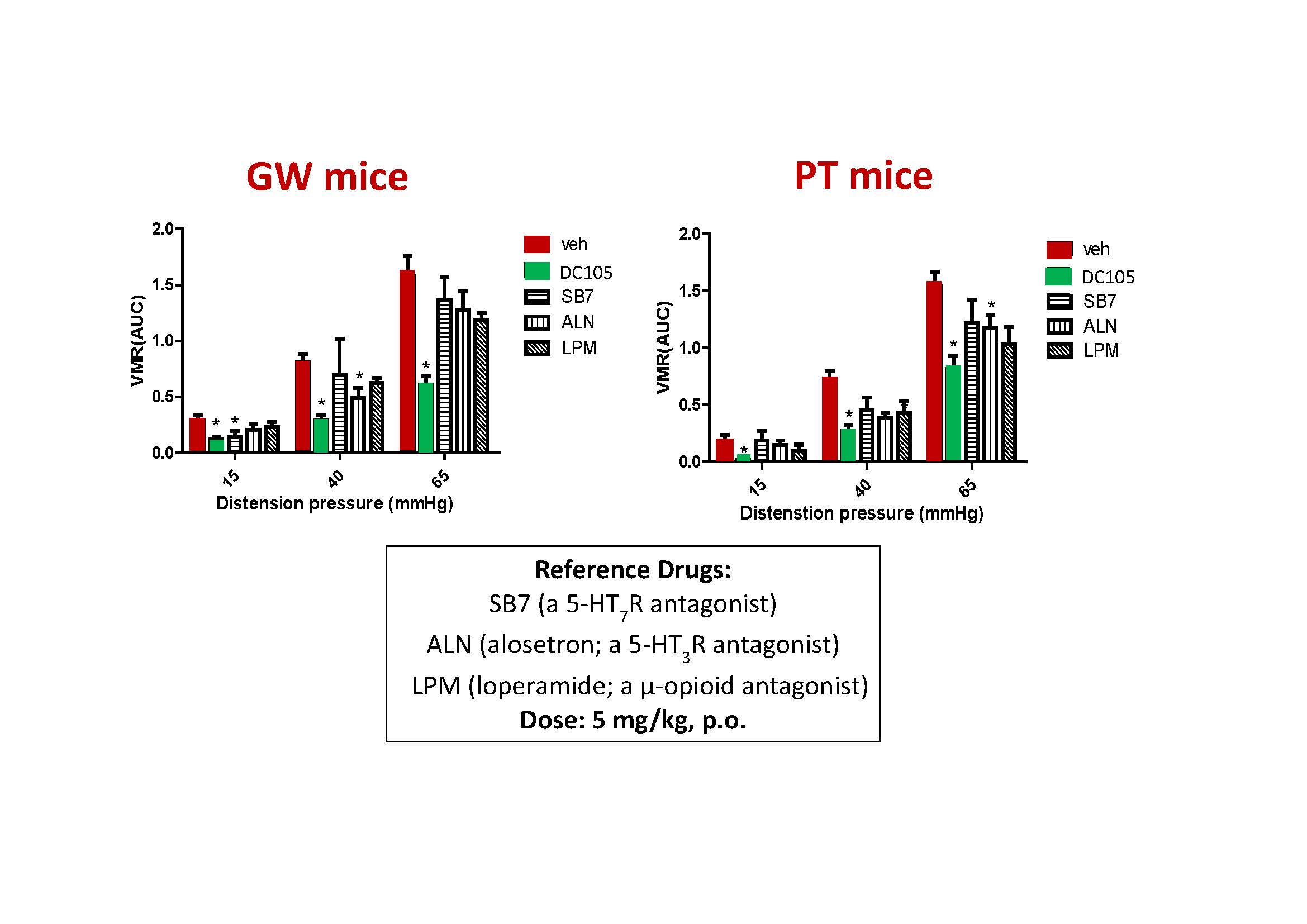
| 技術簡介 | 腸躁症腹痛的治療仍為高度未獲滿足的醫療需求。本技術以血清素第七型受器為治療腸躁症腹痛的新標靶,建立兩種腸躁症動物模式並利用直腸撐張刺激內臟動器反應測定新藥抑制痛覺過敏的能力。經由藥物設計與優化階段,挑選出具高度專一性、口服後有效降低內臟高敏感性、並具有良好安全性的腸躁症腹痛首創型新藥DC105。 |
||
| 科學突破性 | 腸躁症腹痛的治療仍為高度未獲滿足的醫療需求,本團隊研究的目標聚焦於開發安全有效的腸躁症腹痛首創型新藥。成功發展的新技術具有下列重大科學突破性成果:一、發現血清素第七型受器可作為治療腸躁症腹痛的全新生物標靶;二、找出造成腸躁症痛覺過敏的創新藥理機轉;三、開發首創型腸躁症腹痛治療新藥DC105。 |
||
| 產業應用性 | 雖然Rifaximin對腸躁症腹痛的療效有限,2021年的銷售額仍達16.5億美金。DC-105屬於治療腹瀉型腸躁症腹痛首創型小分子新藥,製程經濟易量產,受完整專利保護,具高度市場應用性與競爭力,預期上市後每年銷售額為10~30億美金。下階段將評估DC-105用於治療便秘型腸躁症病人的潛力。 |
||
| 關鍵字 | 腸躁症 腹痛 首創型 臨床前試驗 試驗中新藥 血清素第七型受體 拮抗劑 功能性胃腸道疾病 痛覺過敏 新藥開發 | ||
- 聯絡人
- 張莉德
- 電子信箱
- litechang@ntu.edu.tw