| Technical Name | COVID-19 DiagnosisTherapy | ||
|---|---|---|---|
| Project Operator | Academia Sinica | ||
| Project Host | 吳漢忠 | ||
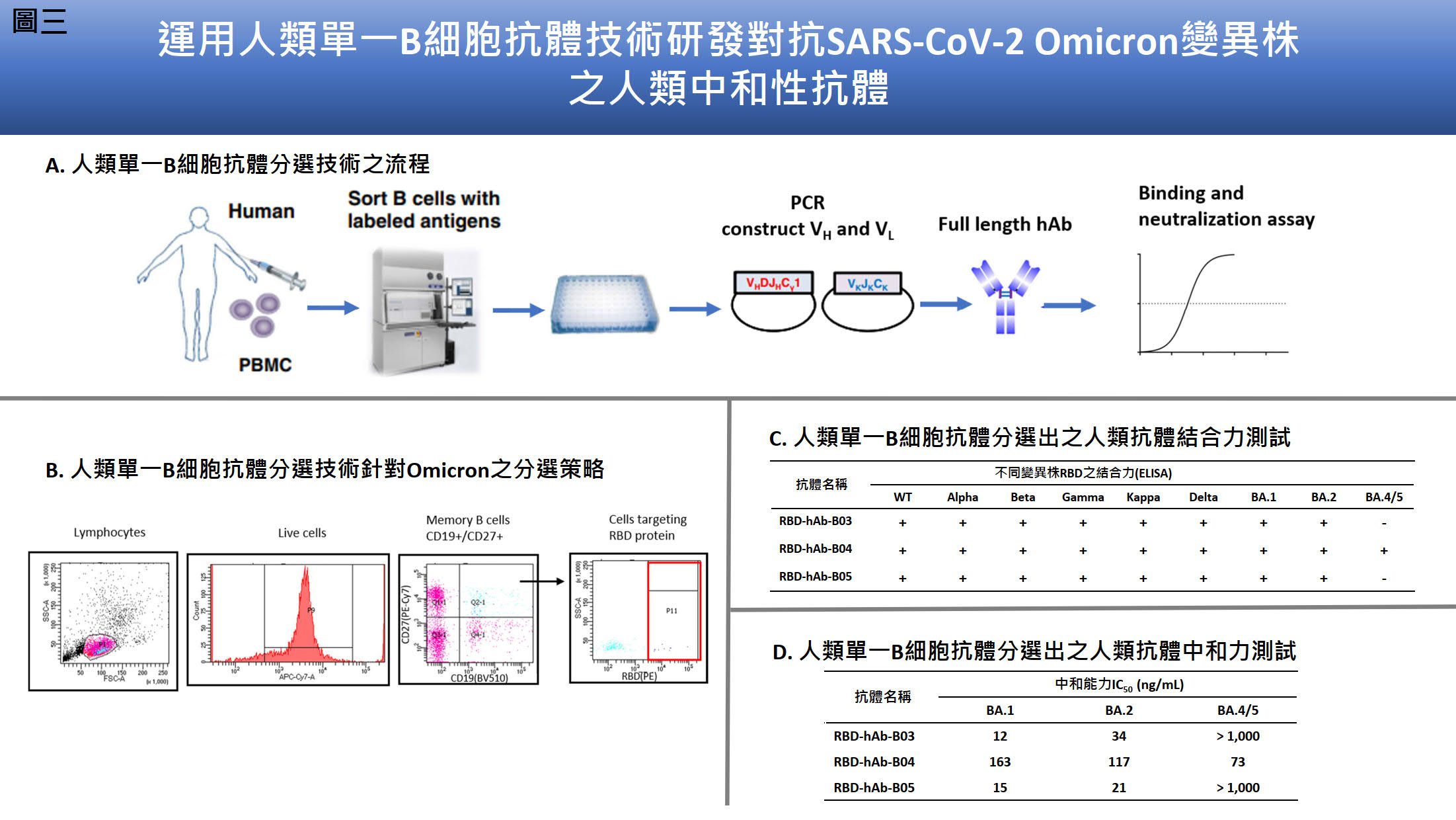
| Summary | For detection of SARS-CoV-2, we generated mAbs against NPused these mAbs to develop the antigen rapid test “Acadecise”. It showed high sensitivity in clinical trialsreceived the EUA from TFDA. To overcome the immune escape of SARS-CoV-2 Omicron variants, we used mRNA-lipid nanoparticle (LNP) immunization method to generate Omicron-targeting mAbs, two of which were engineered to humanized Abs for broadly neutralizing all variants of concern, including Omicron. We further utilized single B cell Ab platform to isolate human Abs (hAbs) against Omicron from vaccinated healthy donors. The hAbs showed the neutralizing potency to Omicron BA.2BA.4/5. The Abs we developed may be useful to detecttreat current SARS-CoV-2 variants. |
||
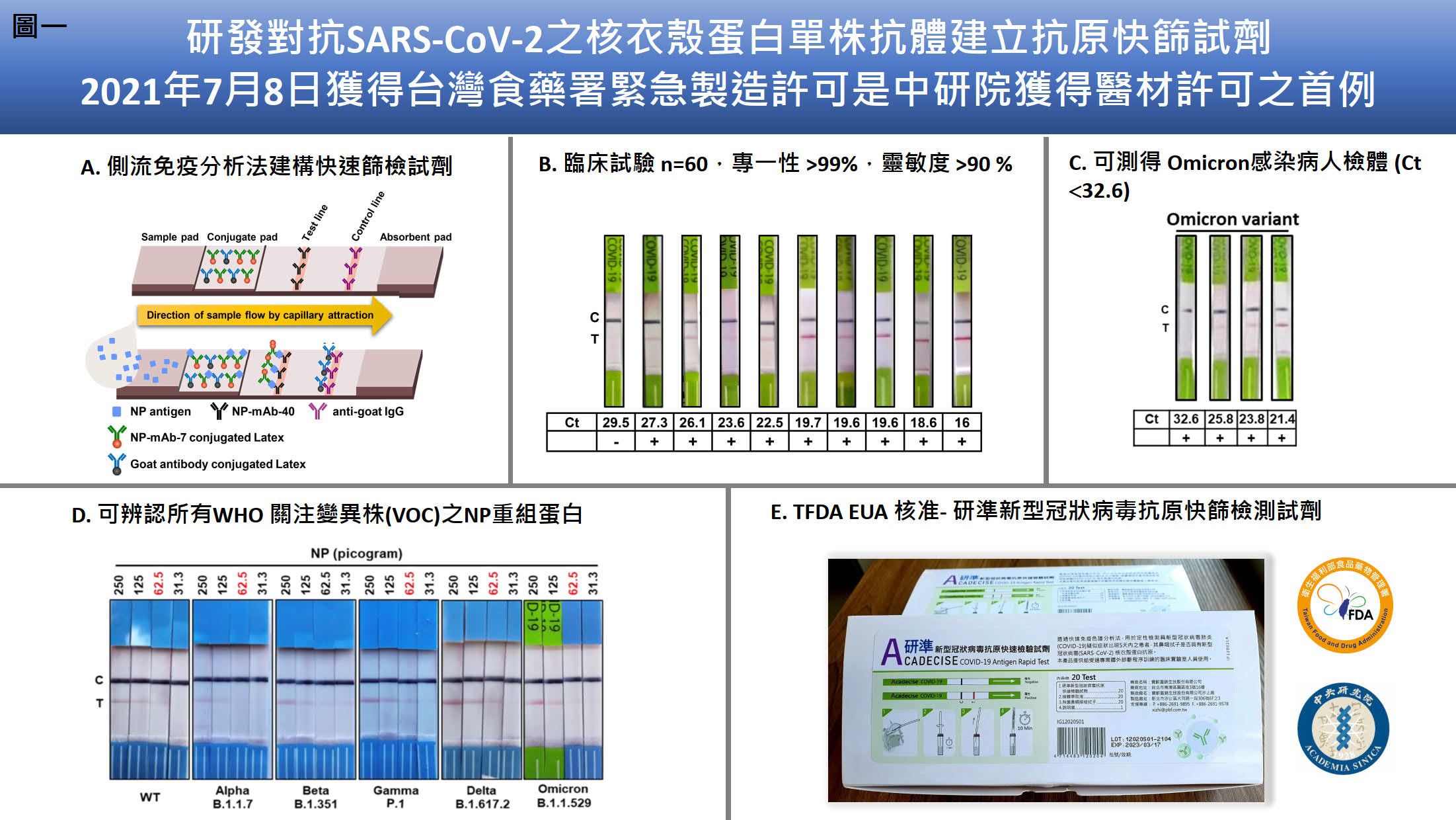
| Scientific Breakthrough | Compared to the sensitivity of the US FDA-approved SARS-CoV-2 antigen rapid tests, Acadecise is listed 6th according to Limit of Detection. Thus, the sensitivity of Acadecise is internationally competitive. More importantly, clinical data showed that Acadecise enabled to detect Omicron-positive nasopharyngeal specimens with quite low virus loads (Ct ≤32.6). LNP-mediated delivery is a key technology of the successful implementation of mRNA-based vaccines. We used this time-saving approach to efficiently stimulate humoral immunityrapidly generate broadly neutralizing Abs against all VOC. We also isolated Omicron-specific human Abs by a human single B Ab platform. The identified Abs exhibit low IC50 values against BA.1, BA.2BA.4/5. |
||
| Industrial Applicability | The SARS-CoV-2 rapid antigen test “Acadecise” has been approved by the Taiwan FoodDrug Administration for emergency manufacturing. This is the first instance in which Academia Sinica has obtained a medical material license. After the manufacturingdistribution contract is signed, the test can be marketed for virus detection. In addition to being used for treatment, broad-spectrum neutralizing antibodies can also be used for prophylaxis, helping immunocompromised individuals to rapidly increase their concentrations of neutralizing antibodiesprevent the occurrence of mild to severe COVID-19. |
||
| Keyword | COVID-19 SARS-CoV-2 neutralizing antibodies antigen rapid test humanized antibodies mRNA-LNP | ||
- Contact
- Ruei-Min Lu
- reminlu@gate.sinica.edu.tw
other people also saw