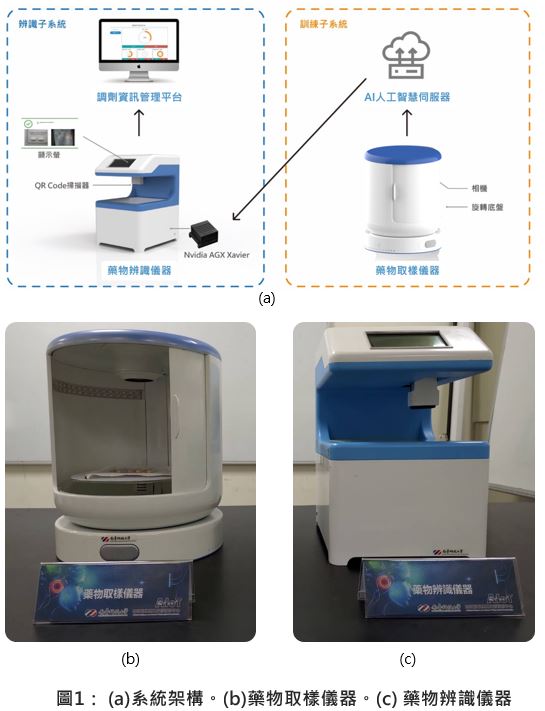
| Summary |
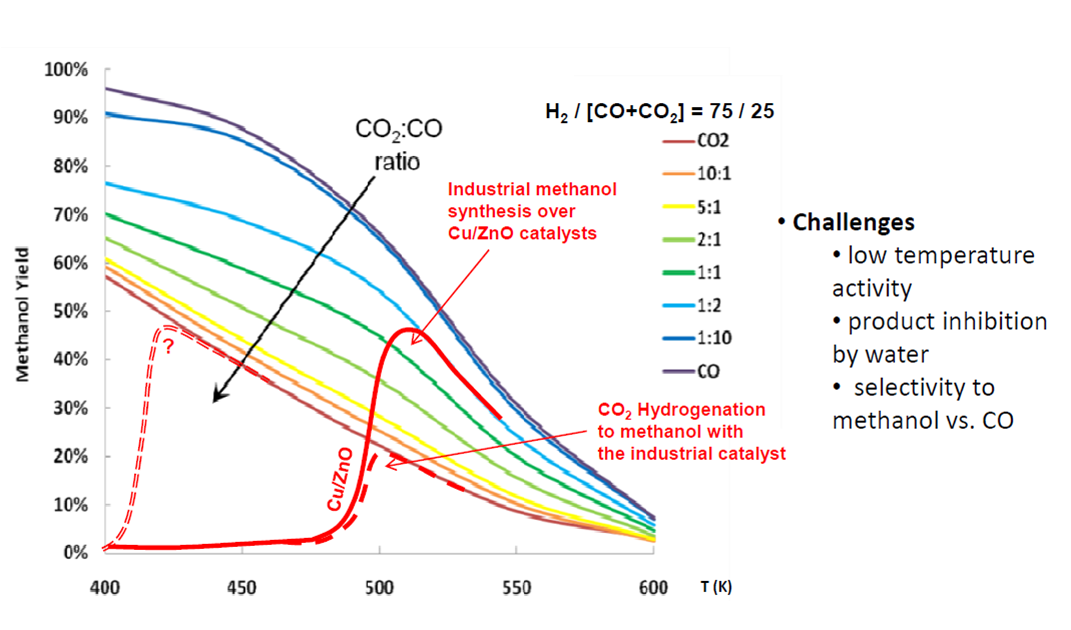
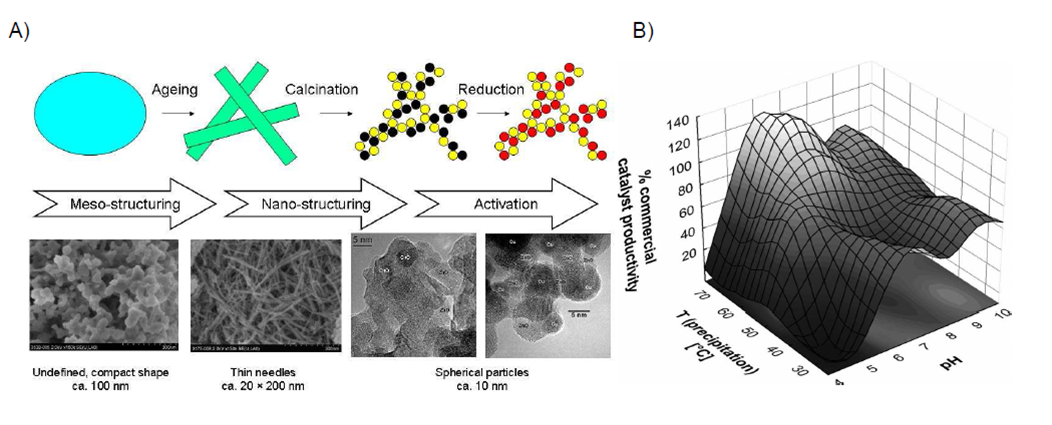
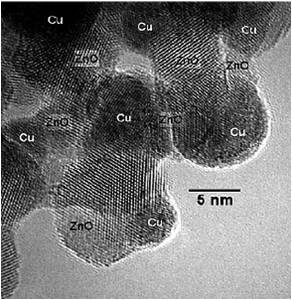
This invention provides a method for synthesis a catalyst for catalyzing hydrogenation of CO2 to methanol. The precipitation process was controlled at 70 oCaging for 1.5 hour. The CuO: ZnO: Al2O3 weight ratios are 7: 3: 1. The particle size of CuO is less than 5 nm. The reaction is carried at 50 bar250 oC, CO2/H2= 1/3. Adding hydrophobic fumed silica, water is expelled from catalyst surface,the reaction can shift to product side. Therefore the CO2 conversion can reach 90 thermodynamic equilibrium conversion, methanol selectivity is greater than 95.
|
| Scientific Breakthrough |
In this invention, we developed a special process to synthesize catalyst with high CuO content (70 wt.)small CuO particle size ( 5 nm). The reaction is carried at 50 bar250 oC, CO2/H2= 1/3. Adding hydrophobic fumed silica, water is expelled from catalyst surface,the reaction can shift to product side. Therefore the CO2 conversion can increase. By using this special catalyst, CO2 conversion can reach 90 thermodynamic equilibrium conversion, methanol selectivity is greater than 95.
|
| Industrial Applicability |
This invention can be used on CO2 utilization to methanol. CO2 hydrogenation is a thermodynamic-controlled reactionthe activation energy is very high. It is difficult to reach thermodynamic equilibrium conversion at low temperature. We have developed a catalyst which can reach 90 equilibrium conversion at 250 oC50 bar. This invention can be used in any semiconductor, steel industry,petrochemical industry. Since methanol is a feedstock for many processes, one can use this process to reach the target of carbon neutralcyclic economy.
|