| 技術名稱 | BPR1R系列:新穎集落刺激因子1受體抑制劑作為癌症免疫調節劑 | ||
|---|---|---|---|
| 計畫單位 | 國家衛生研究院 | ||
| 計畫主持人 | 謝興邦 | ||
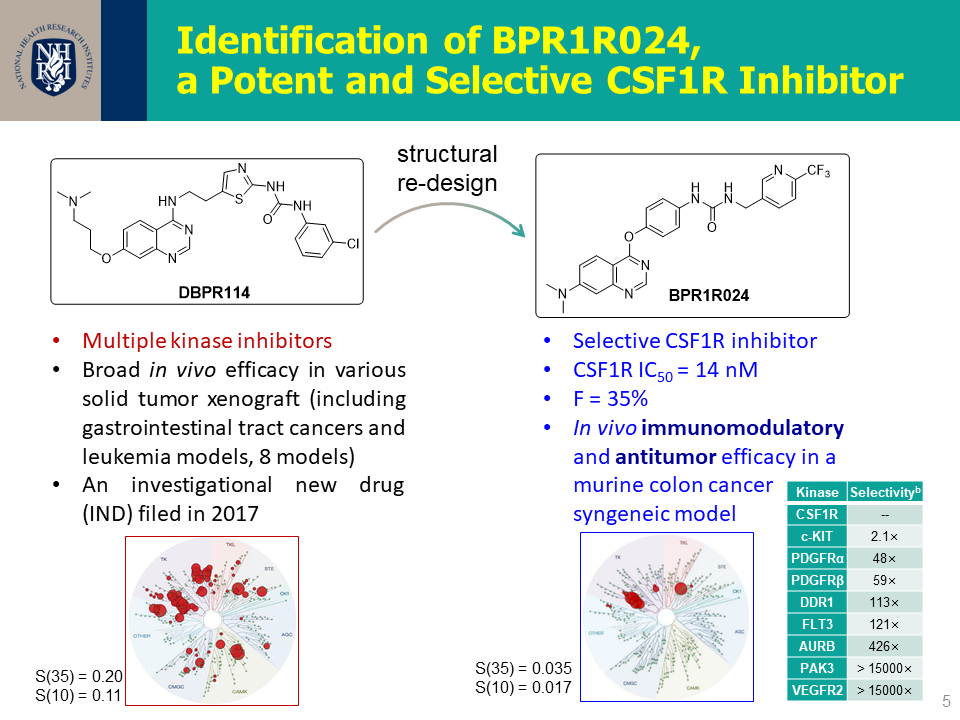
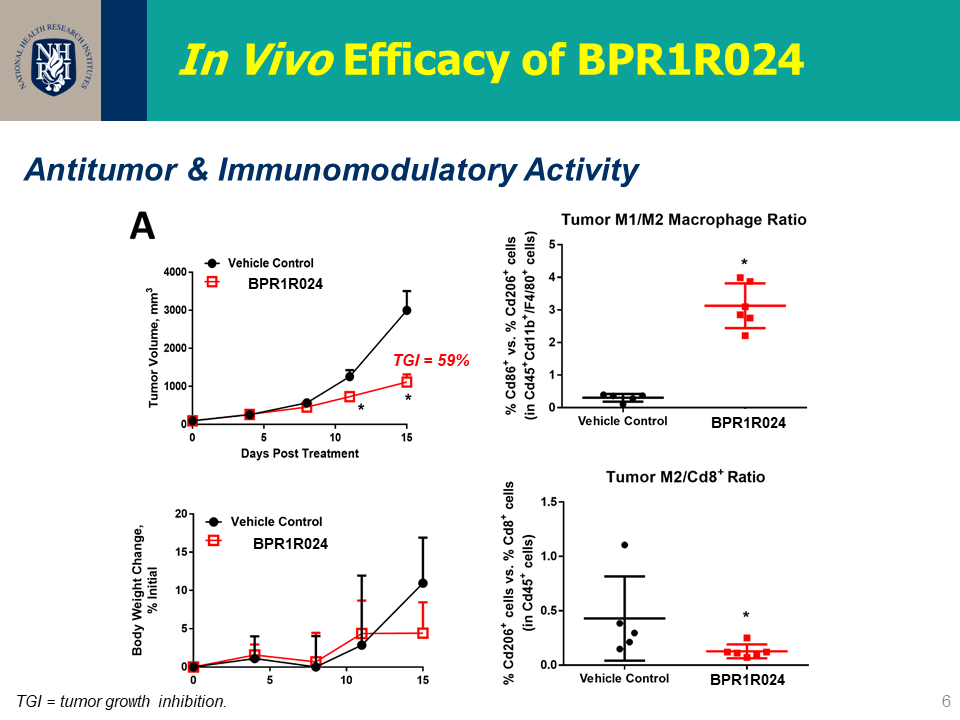
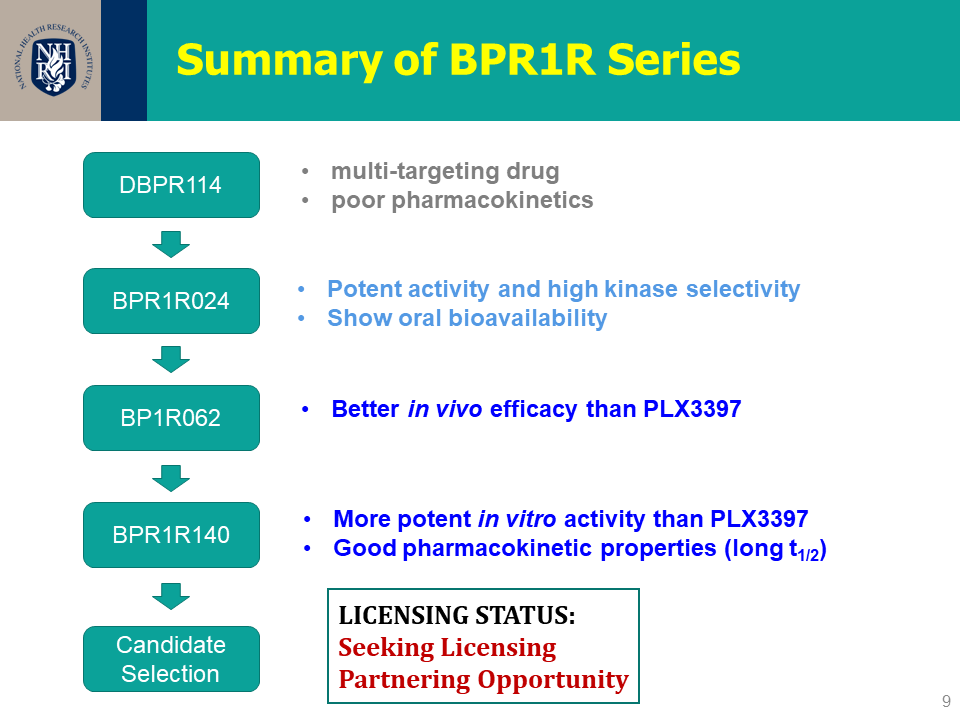
| 技術簡介 | 本團隊發展一系列的CSF1R抑制劑,包含超過160個新穎化合物。其中幾個重要的先導化合物具高選擇性及優秀口服吸收能力,可有效抑制老鼠大腸癌腫瘤細胞生長,改善體內M1/M2型巨噬細胞之比例並逆轉免疫抑制之腫瘤微環境,並且證明有穿透血腦屏障的能力。此發明於2020年已申請美國、台灣以及PCT專利。 |
||
| 技術影片 | |||
| 科學突破性 | "腫瘤生長抑制率69;相同劑量下pexidartinib為57。 穿透血腦屏障的能力: B/P ratio = 31 合成步數短(5-7步內),已完成10克級之合成,利於CMC之量產 化合物口服可利用率可達到38 優異的激酶選擇性,S(35) = 0.035 @ 1 uM" |
||
| 產業應用性 | "CSF1R激酶抑制劑的全球市場價值於2024年將來到238億美元 免疫治療:合併使用PD-1單株抗體或是細胞毒性藥物顯示具有增效作用。本團隊開發的CSF1R抑制劑具有高選擇性低毒性等特性,減少合併使用藥物之風險。 治療阿茲海默症和帕金森氏症的潛力:我們的化合物具有優良的腦穿透能力可用於此類疾病治療 |
||
| 媒合需求 | 天使投資人、策略合作夥伴 |
||
| 關鍵字 | 集落刺激因子1受體抑制劑 癌症治療 免疫治療 激酶抑制劑 免疫調節劑 腫瘤微環境 腫瘤相關巨噬細胞 癌症免疫治療 集落刺激因子1受體 聚落刺激因子1受體 | ||
- 聯絡人
- 李昆鴻
- 電子信箱
- quin22805@nhri.edu.tw