:::
- 首頁
- /
- 年度
- /
- 2021
- /
- 精準健康(生技&新藥)
- /
- 抗癌小分子新藥之臨床前開發與精準治療策略
| 技術名稱 | 抗癌小分子新藥之臨床前開發與精準治療策略 | ||
|---|---|---|---|
| 計畫單位 | 臺北醫學大學 | ||
| 計畫主持人 | 潘秀玲 | ||
| 技術簡介 | 本團隊技術 MPT0G211為一高度選擇性 HDAC6 抑制劑,受到完整專利保護,主要針對多發性骨髓瘤與腦癌適應症進行開發,現今已完成大部分的臨床前試驗,僅剩狗 28 天長期毒理試驗即申請美國和台灣 IND新藥審查,預計 2022 年 Q2 申請 IND。 |
||
| 技術影片 | |||
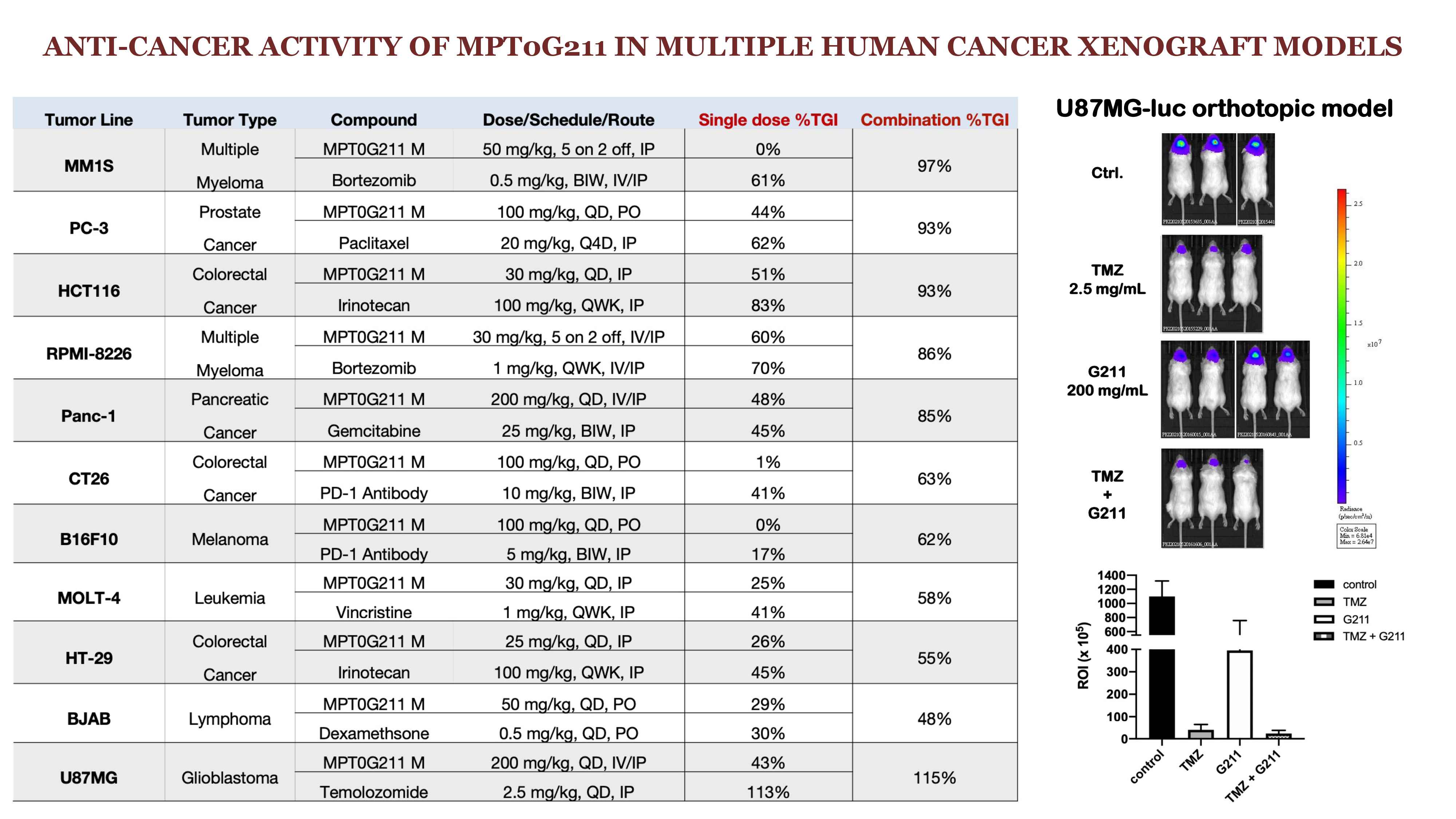
| 科學突破性 | MPT0G211對HDAC6活性抑制優於各界研發的HDAC6抑制劑. 在肺癌與腦癌末期病患之循環腫瘤細胞(CTC), MPT0G211可顯著抑制CTC存活, 顯示MPT0G211對抗藥性與復發性肺癌與腦癌具有開發潛力, 未來可以orphan drug designation program加速開發. |
||
| 產業應用性 | HDAC6抑制劑廣泛開發於癌症, 為國際藥廠開發之重點標的. MPT0G211證實於抗藥性與復發性腦癌具有開發潛力. 已獲中研院國家生技研究園區轉譯計畫支持, 預計2022 Q2申請美國與台灣 IND. |
||
| 媒合需求 | 天使投資人、策略合作夥伴 |
||
| 關鍵字 | MPT0G211 中研院國家生技研究園區 小分子抑制劑 組蛋白去乙醯酶六 新藥開發 腦癌 癌症 臨床前試驗 IND 第一期臨床試驗 | ||
- 聯絡人
- 黃瀚立
- 電子信箱
- hlhuang@tmu.edu.tw