| Technical Name | MRI Compatibility Testing and Evaluation Platform for Implantable Medical Device | ||
|---|---|---|---|
| Project Operator | Institute of Biomedicall Engineering and Nanomedicine, National Health Research Institutes | ||
| Project Host | 郭立威 | ||
| Summary | Within the MRI environment, strong magnetic fields and radiofrequency pulses may introduce potential safety risks for medical devices. Consequently, the evaluation of MRI compatibility for implantable medical devices has become a critical focus in recent medical device development. Our laboratory has established a comprehensive platform for MRI compatibility testing and evaluation. The testing procedures are conducted in accordance with ASTM standards, and we are able to provide test reports that comply with ISO 17025 requirements. This enables manufacturers to rigorously assess both the MRI compatibility and safety of their products. |
||
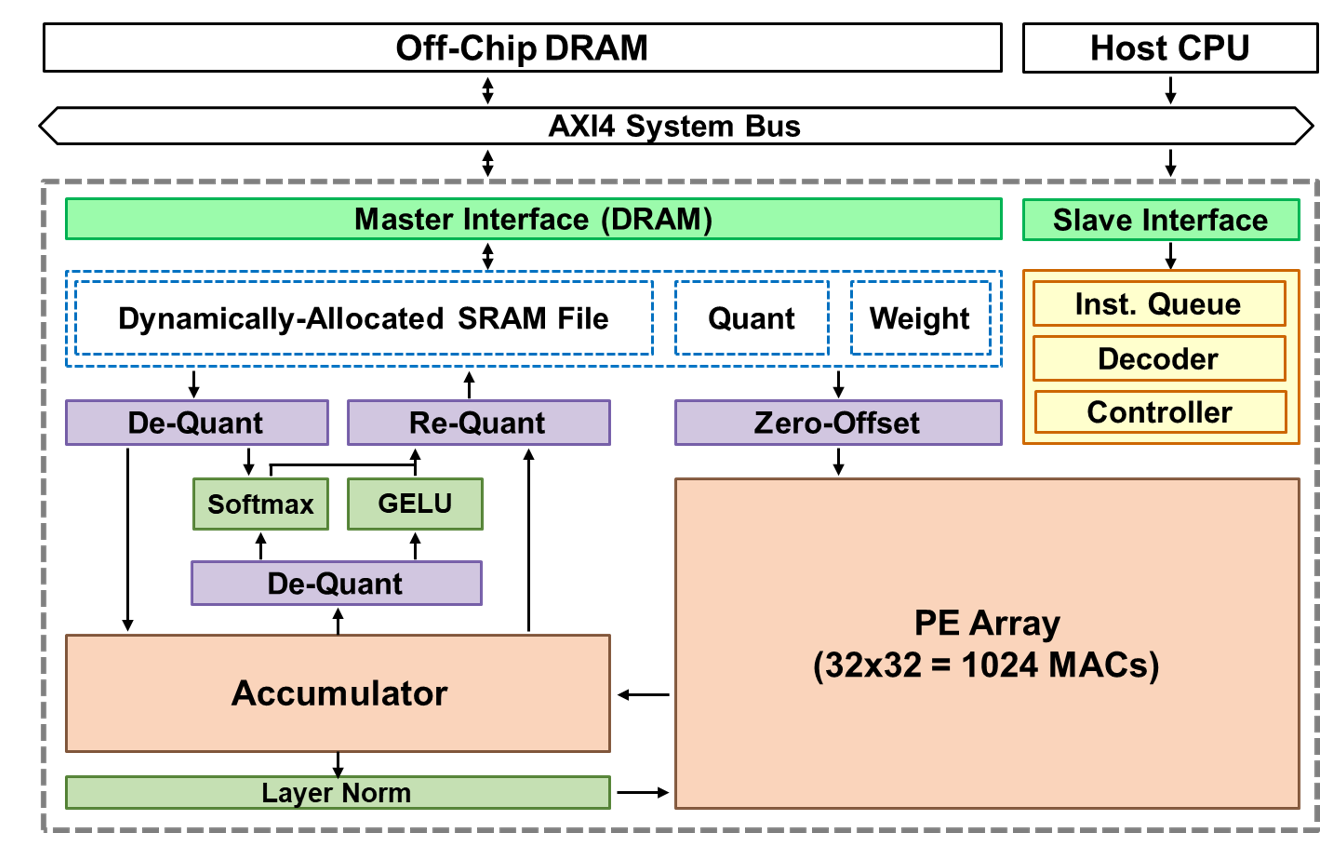
| Scientific Breakthrough | This implantable medical device testing platform integrates experimental validation within MRI systems and advanced electromagnetic simulation techniques. It enables the evaluation of the displacement, rotation, thermal effects, and imaging artifacts under magnetic fields of the target medical device. Beyond characterizing these properties, the platform provides substantial benefits in enhancing the safety of implantable medical devices in MRI environments. |
||
| Industrial Applicability | We have successfully established this testing platform, thereby strengthening the MRI compatibility of implantable medical devices. The platform markedly improves the safety of such devices in MRI environments, while simultaneously facilitating domestic academic, industrial, and research institutions in obtaining regulatory approvals from international authorities. Consequently, it enhances both the global competitiveness and the intrinsic value of these medical device products. |
||
- Contact
- Li-Wei Kuo
- lwkuo@nhri.edu.tw
other people also saw