| Technical Name | Hepatitis B virus detection platforms for novel biomarkersclinical applications | ||
|---|---|---|---|
| Project Operator | National Cheng Kung University | ||
| Project Host | 黃溫雅 | ||
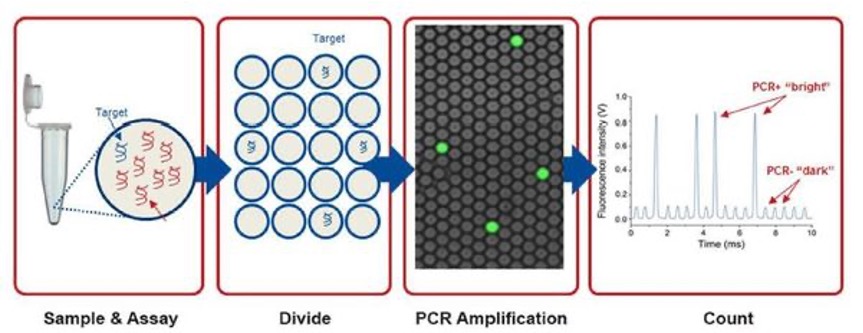
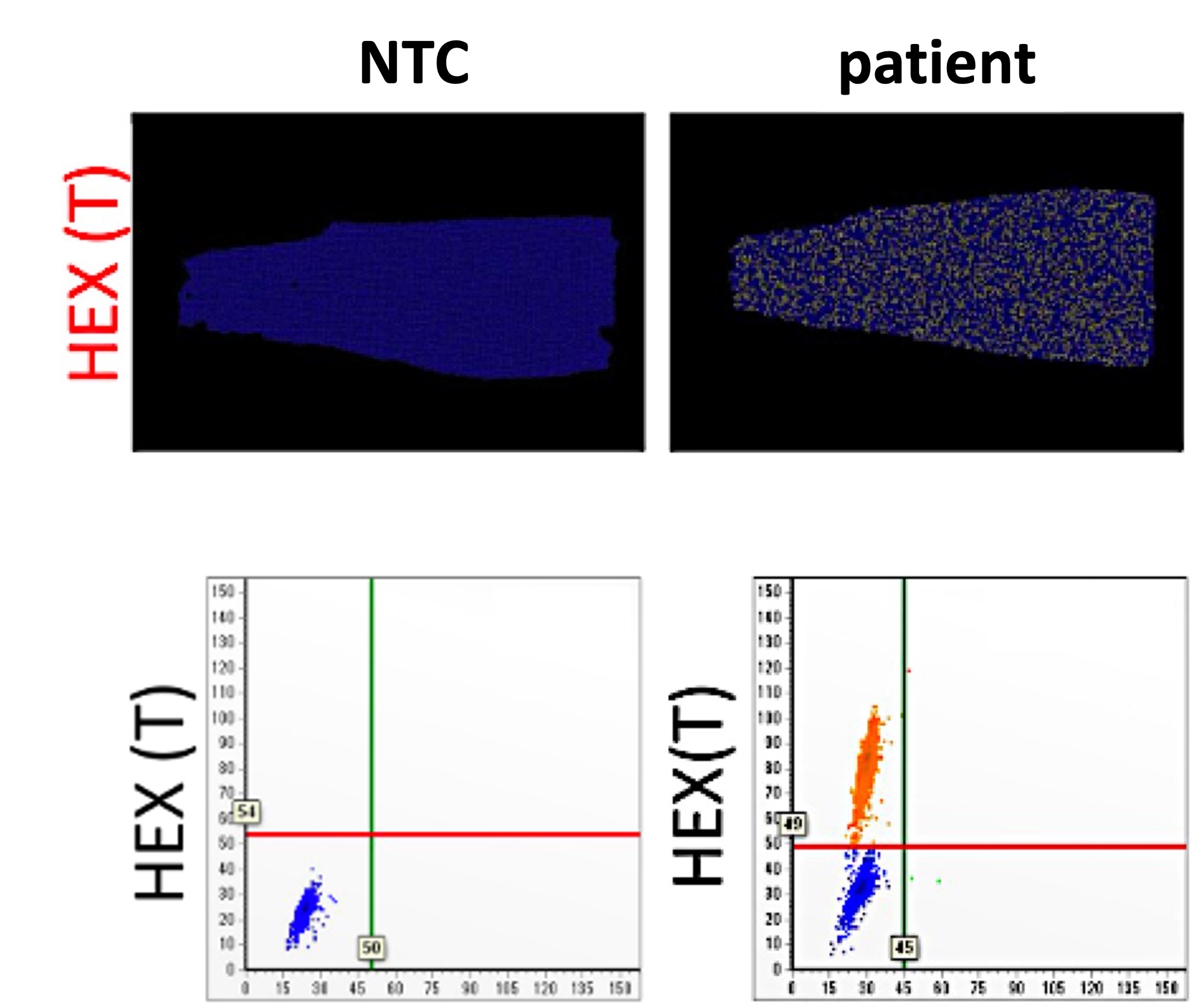
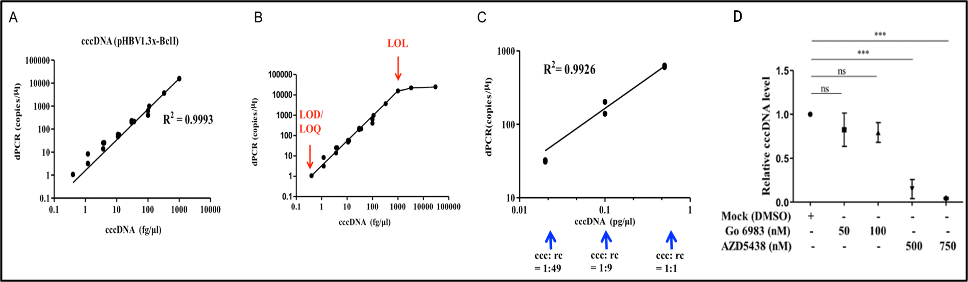
| Summary | We developed the purification protocols of clinical specimen cccDNA and, improved the stabilitydetection specificity of the cccDNA. We also developed a dPCR system for high-sensitivity quantifying HBV DNA. Moreover, a LDTS test kit for simultaneous quantification of HBV viral loadcccDNA by dPCR has been established. Clinical tests in HBV cohorts were performed to screen out low-titer HBV-positive carriers. Large-scale screening for the low-titer HBV carriers by dPCR is in progress. |
||
| Scientific Breakthrough | 1. Optimization of the cccDNA purification process to standardize the clinical sample extraction protocols.2. A dPCR system to improve the sensitivity of HBV detection to screen out the low-titer HBV carriers and, to evaluate antiviral strategies.3. A LDTS test for dPCR simultaneous quantification of HBV viral loadof cccDNA as predictive biomarkers of hepatitis recurrenceHCC.4. Application in the development of new antiviral drugs by measurement of the HBV replication products. |
||
| Industrial Applicability | Market value: The market size of hepatitis B therapeutic drugs is predicted to grow to US$471.49 million by 2026. Our developed products can be used in the countries where HBV is prevalenthave high market value.Clinical application: The high-sensitivity dPCR technology to detect HBV viral loadcccDNA can serve as biomarkers of the efficacy of HBV treatments. Through the analysis of clinical samples, we can screen out low-level HBV carriersre-evaluate their treatment strategies. |
||
| Keyword | hepatitis B virus hepatoma precision medicine biomarker molecular diagnosis digital polymerase chain reaction ELISA diagnosis kit anti-viral drug LDTS | ||
- Contact
- Yun-Ping Lee
- fishskin816@gmail.com
other people also saw