| Technical Name | Generation and differentiation of universal induced pluripotent stem cells for stem cell therapy | ||
|---|---|---|---|
| Project Operator | National Central University | ||
| Project Host | 樋口亞紺 | ||
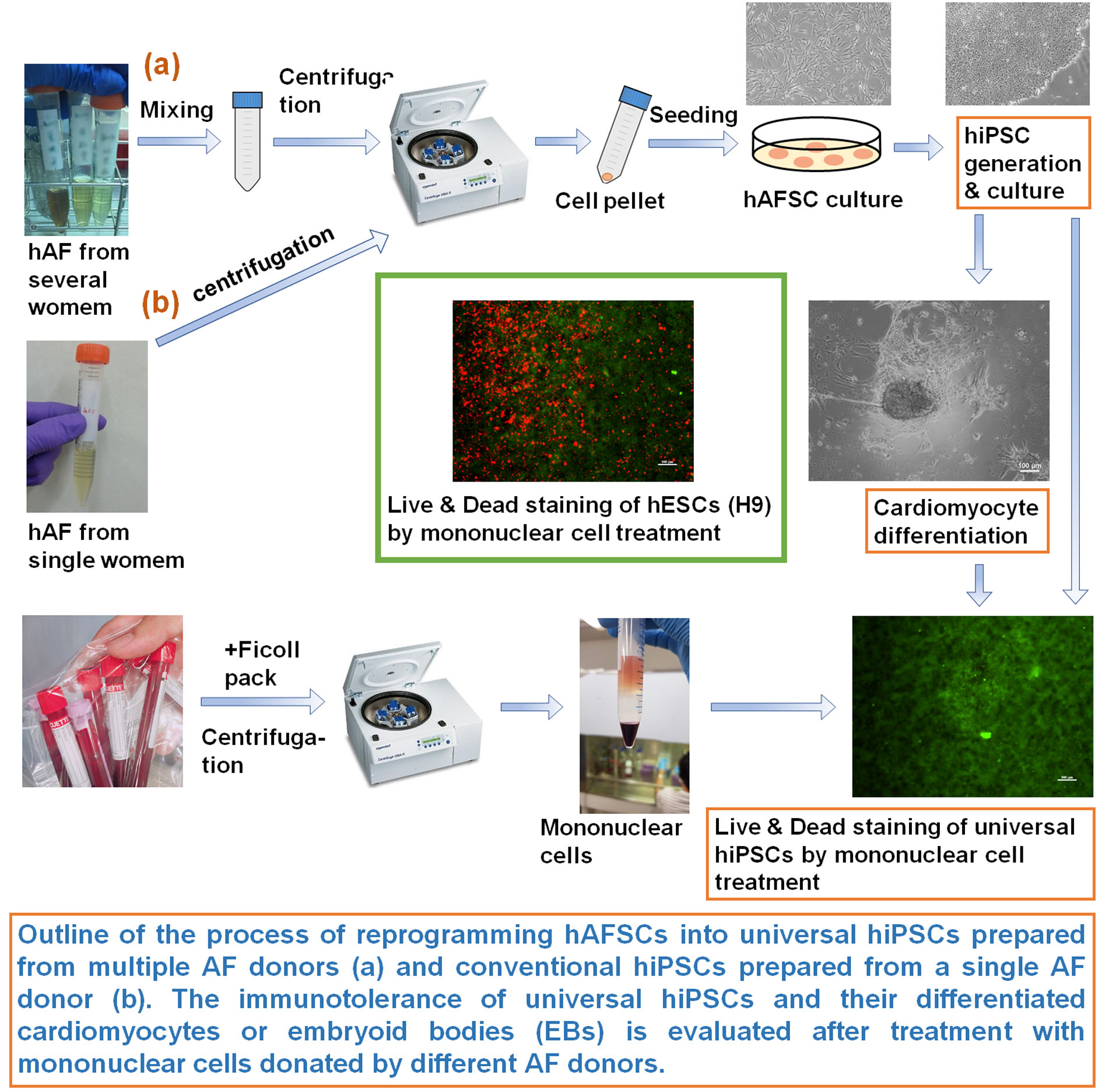
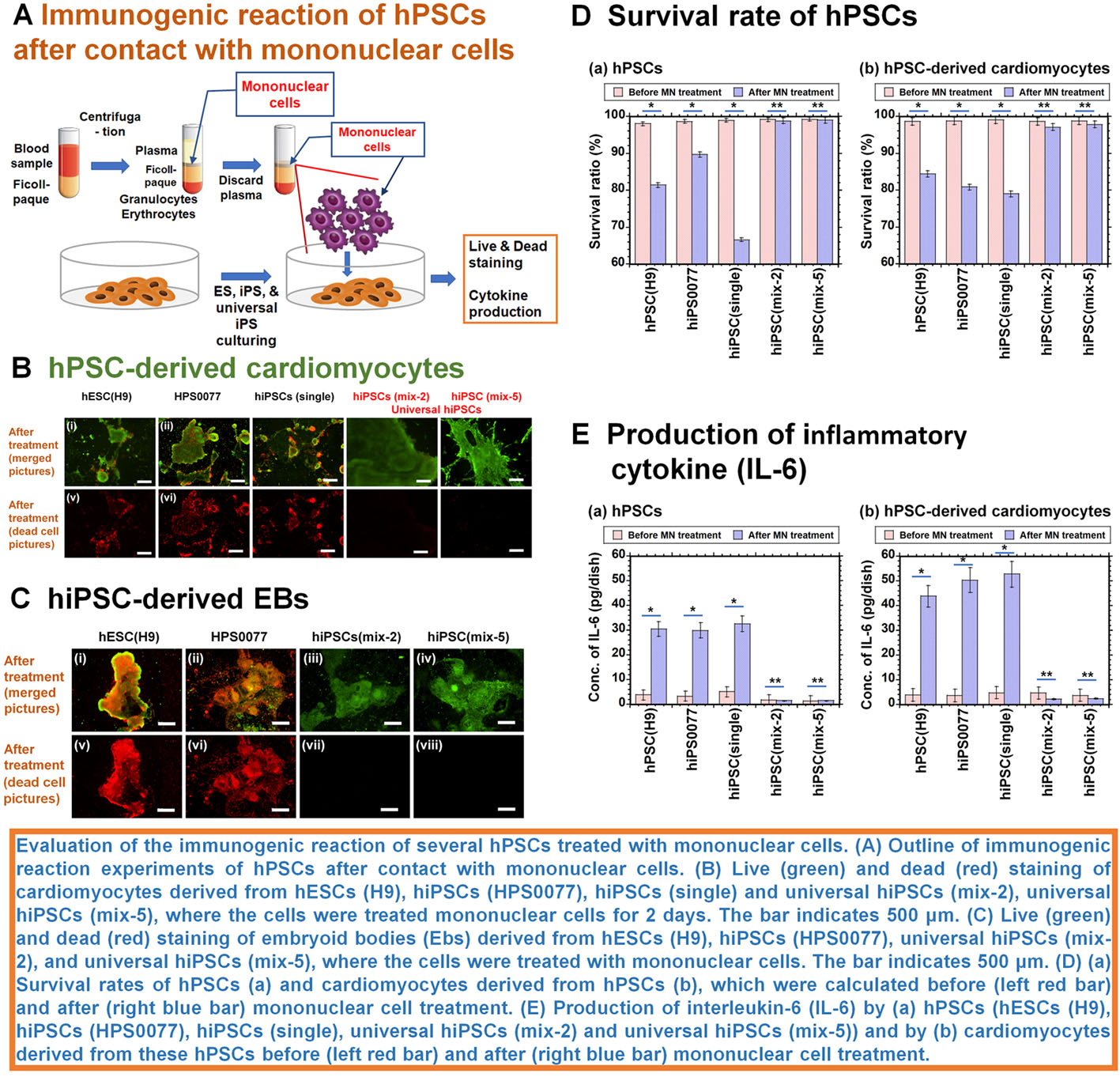
| Summary | We invented universal human induced pluripotent stem cells (hiPSCs) without gene editing; these cells do not express human leukocyte antigen (HLA) Class I and Class II even after differentiation and can theoretically be used to treat any patient using only one cell line. We prepared universal hiPSC with mixing more than two types of amniotic fluid (AF) derived from different donors, and the cells in AF were reprogrammed into hiPSCs, which expressed no HLA Class I and Class II, even after differentiation into cardiomyocytes, embryoid bodies (progenitor cells derived from three germ layers), and mesenchymal stem cells (universal hiPSCs). Cardiomyocytes and embryoid bodies differentiated from universal hiPSCs survived and continued beating even after treatment with mononuclear cells. The mechanism underlying the generation of universal hPSCs is that fetal stem cells contain a specific group of cells that do not express HLA Class I and Class II after reprogramming into hiPSCs. |
||
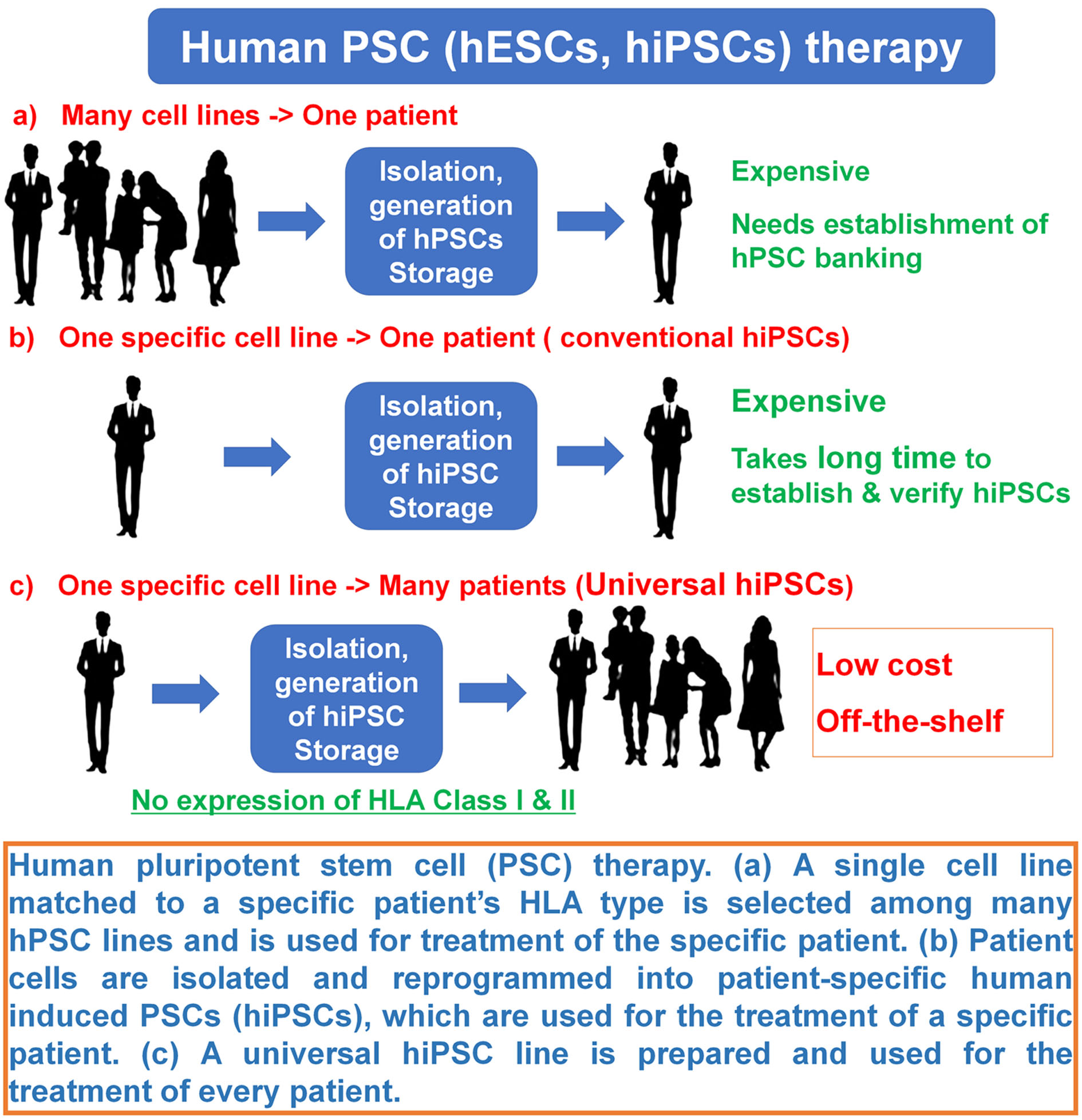
| Scientific Breakthrough | We succeeded to generate universal hiPSCs even after their differentiation (no immune-rejection) without genetic editing and expected no immune-rejection in their transplantation into patients. On the other hand, conventional cell therapy using hiPSCs and human embryonic stem cells (human pluripotent stem cells) can be only used for the patients matching HLA (human leukocyte antigen) Class I and Class II. Current preparation method of universal human pluripotent stem cells needs genetic modification such as knockout B2M (Beta-2 microglobulin) or knockout HLA-B as well as knock out HLA Class I and Class II. We succeeded to generate universal hiPSCs without genetic editing and expected no immune-rejection in their transplantation into patients. |
||
| Industrial Applicability | Currently, the banking of human pluripotent stem cell lines (hPSCs), which are clinically approved, is necessary to provide a source of hPSCs for stem cell therapy. However, 150-170 hPSC lines should be prepared at minimum for the banking of hPSCs currently, which leads to a high cost in the National Insurance Budget, whereas only one cell line of our universal human induced pluripotent stem cells (hiPSCs) is enough and can be used for treatment of every patients using stem cell therapy because of no expression of HLA Class I and Class II even after their differentiation. Our universal hiPSCs can be generated without genetic modification, which can be used much safer compared to conventional universal hiPSCs prepared by genetic modification. |
||
| Keyword | Universal stem cells Human pluripotent stem cells Differentiation Human leukocyte antigen Stem cell Therapy Immune rejection Cell transplantation Regenerative medicine Tissue engineering Stem cell bank | ||
- milk.cat35@gmail.com
other people also saw