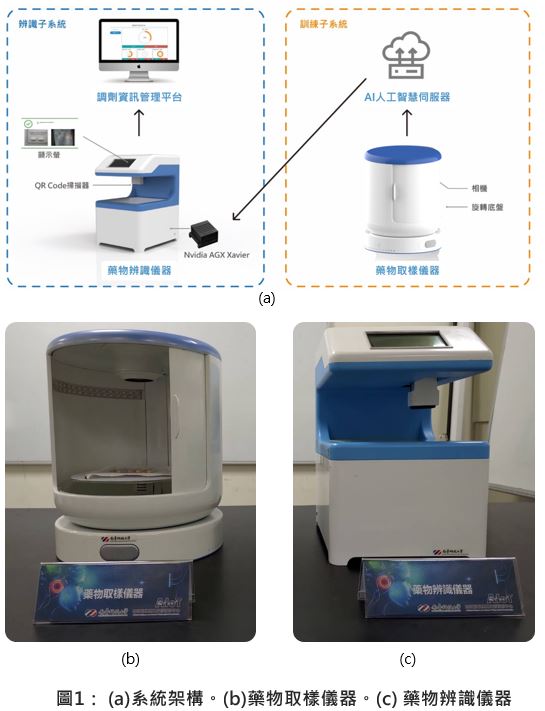
| 技術名稱 | 玻尿酸水膠雛型品試製應用於關節軟骨缺損重建 | ||
|---|---|---|---|
| 計畫單位 | 高雄醫學大學 | ||
| 計畫主持人 | 何美泠 | ||
| 技術簡介 | 由於各種運動損傷及肥胖人口的日趨普及,造成關節軟骨缺損的病人不斷增加,全球膝關節軟骨修復的市場在2014年時約為160億美元,預估其市場價值在2023年將會達到270億美元。 |
||
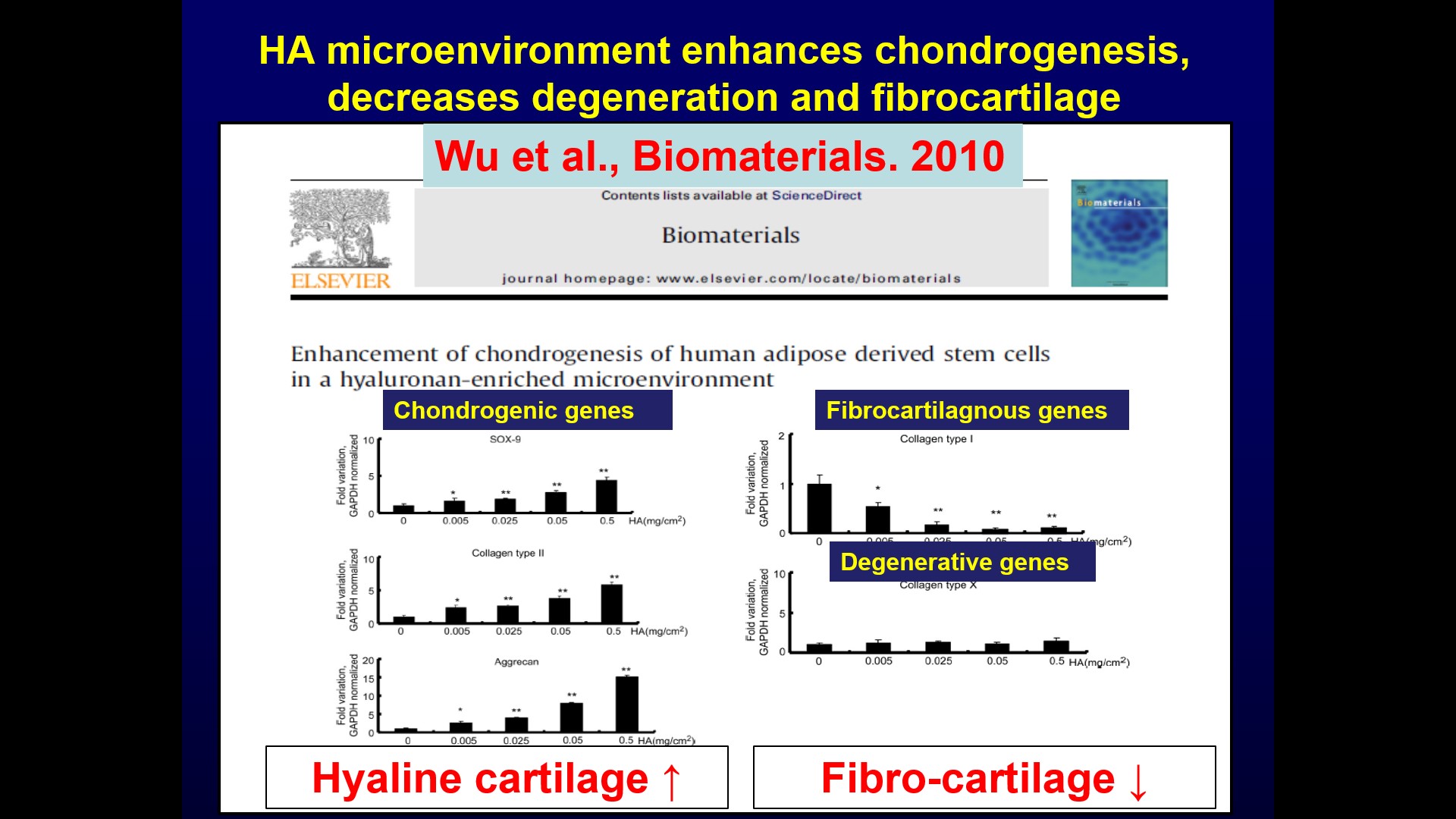
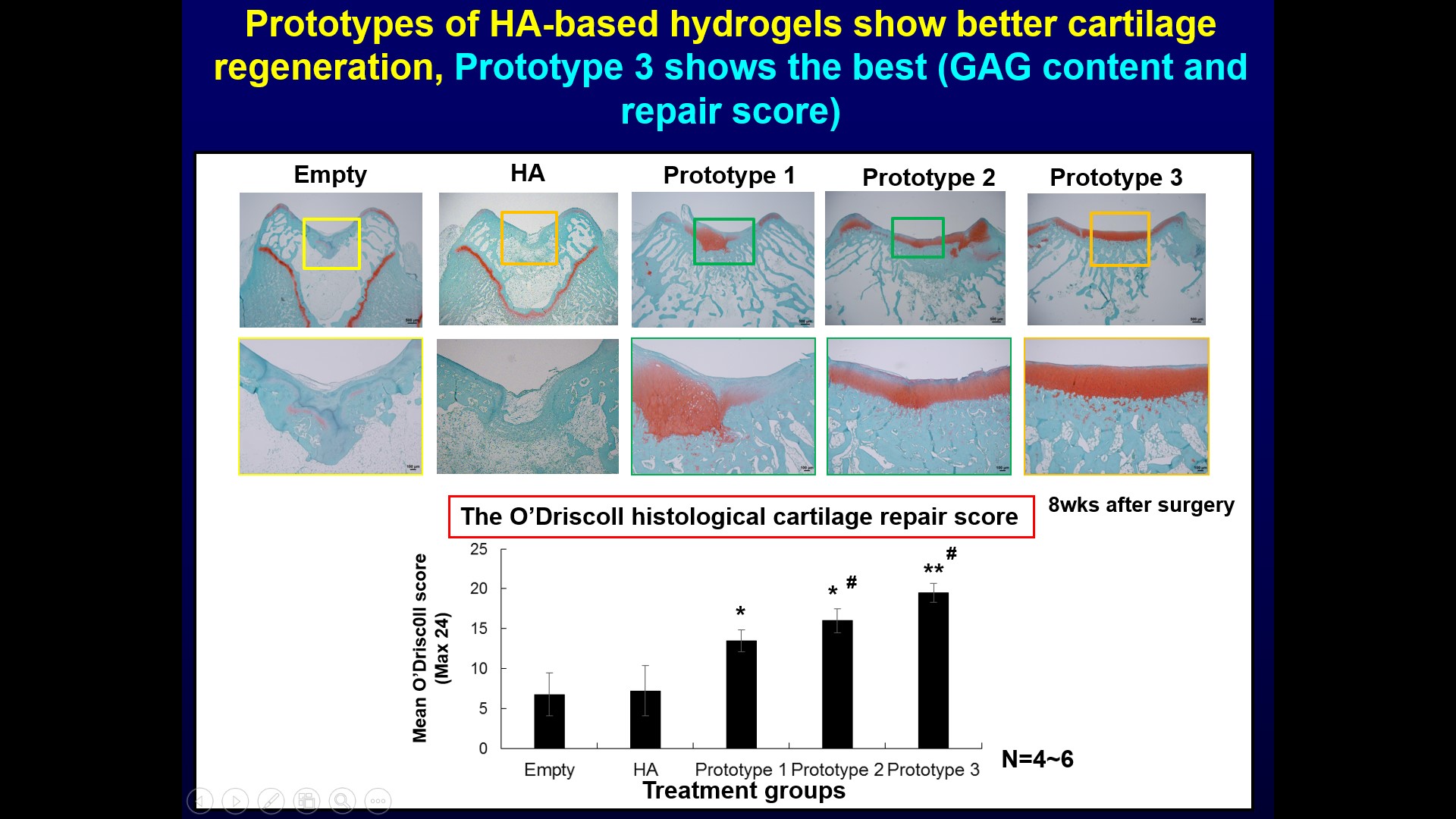
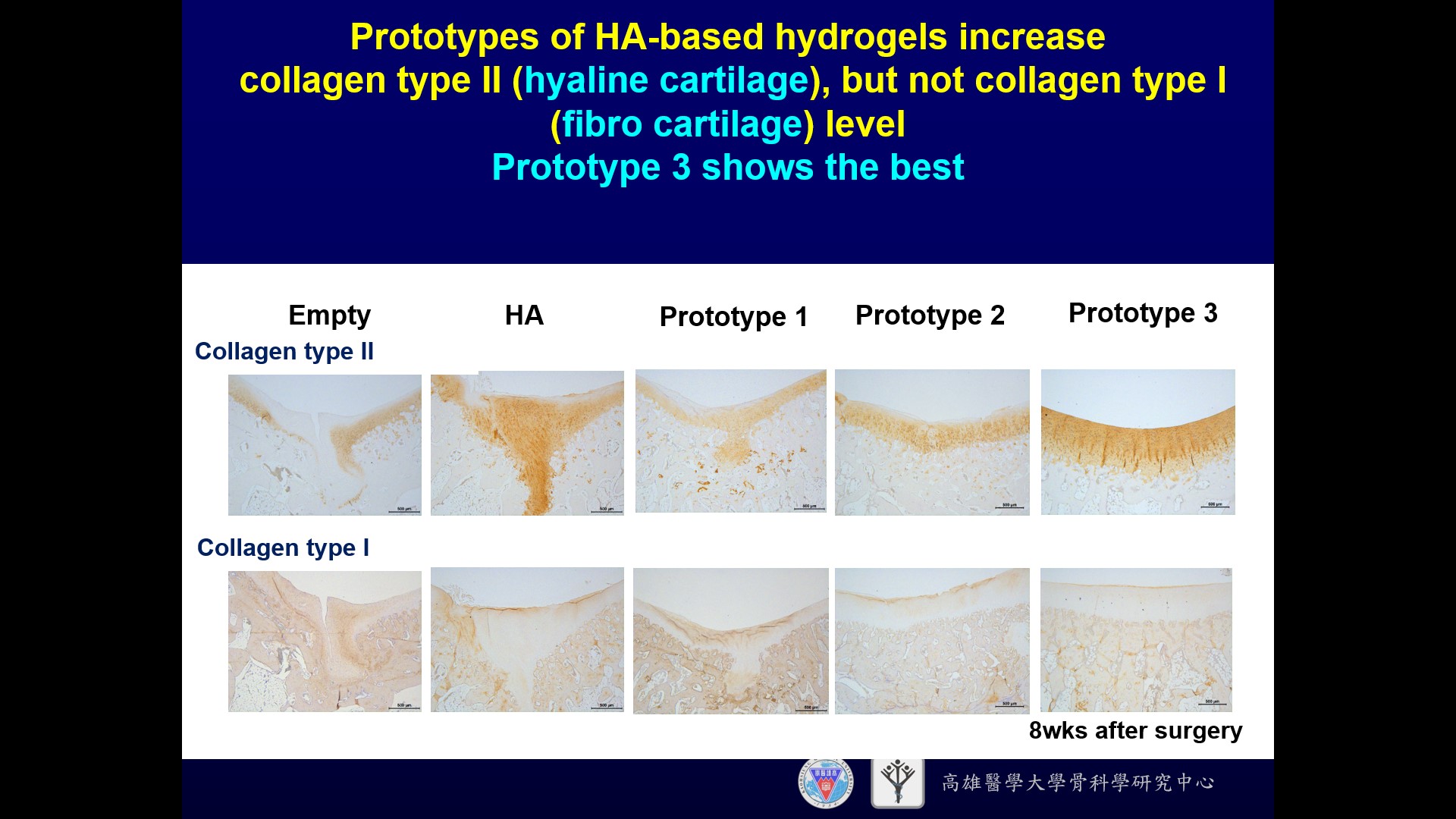
| 科學突破性 | 本技術是交聯玻尿酸水膠技術,可不需配合軟骨細胞或間質幹細胞。相較於其它臨床所使用的微骨折產品,本技術的優勢在於玻尿酸即為關節軟骨及關節液的主要成份,且具備促進透明軟骨形成,減少纖維軟骨形成的功能,再者玻尿酸為關節軟骨主要成份,造成免疫排斥的可能性較小。 |
||
| 產業應用性 | 本產品的目標客戶是美國、中國、歐盟及日本等醫療器材主要的市場之中,由於關節軟骨缺損的臨床病人,由骨科醫師診斷確診後,於膝關節手術之中使用。本技術目標將技術移轉之方式,將此技術移轉具備玻尿酸相關醫療器材生產能力之廠商,包含中華民國、日本或美國之廠商,接續進行生產及銷售。 |
||
| 關鍵字 | 玻尿酸 玻尿酸交聯 軟硬度 透明軟骨 纖維軟骨 幹細胞 微骨折 無細胞 關節液 間質幹細胞 | ||
- 聯絡人
- 吳順成
- 電子信箱
- shunchengwu@hotmail.com
其他人也看了