| Technical Name | Development of prototypes of hyaluronan modified hydrogels for articular cartilage defect regeneration | ||
|---|---|---|---|
| Project Operator | Kaohsiung Medical University | ||
| Project Host | 何美泠 | ||
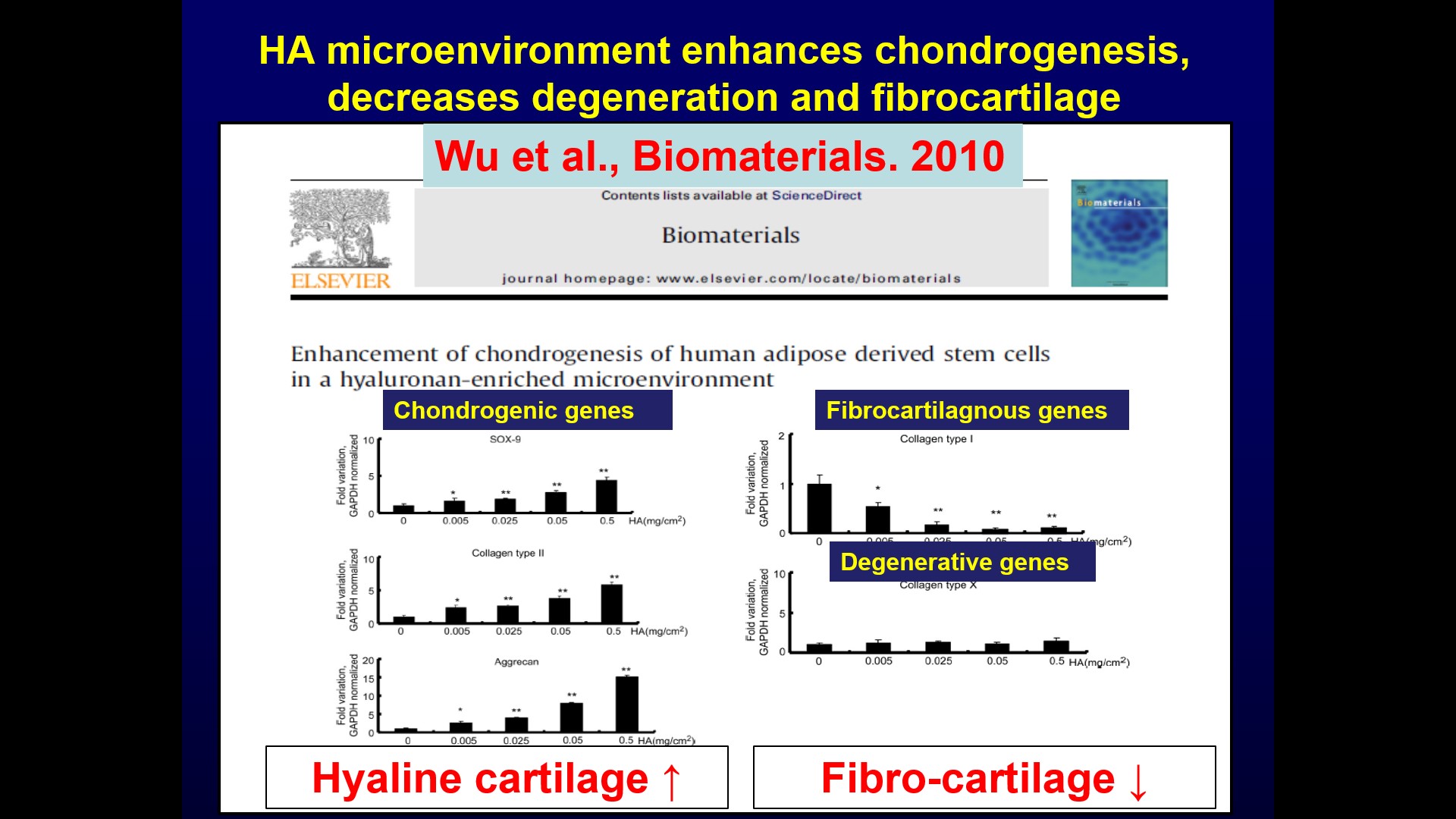
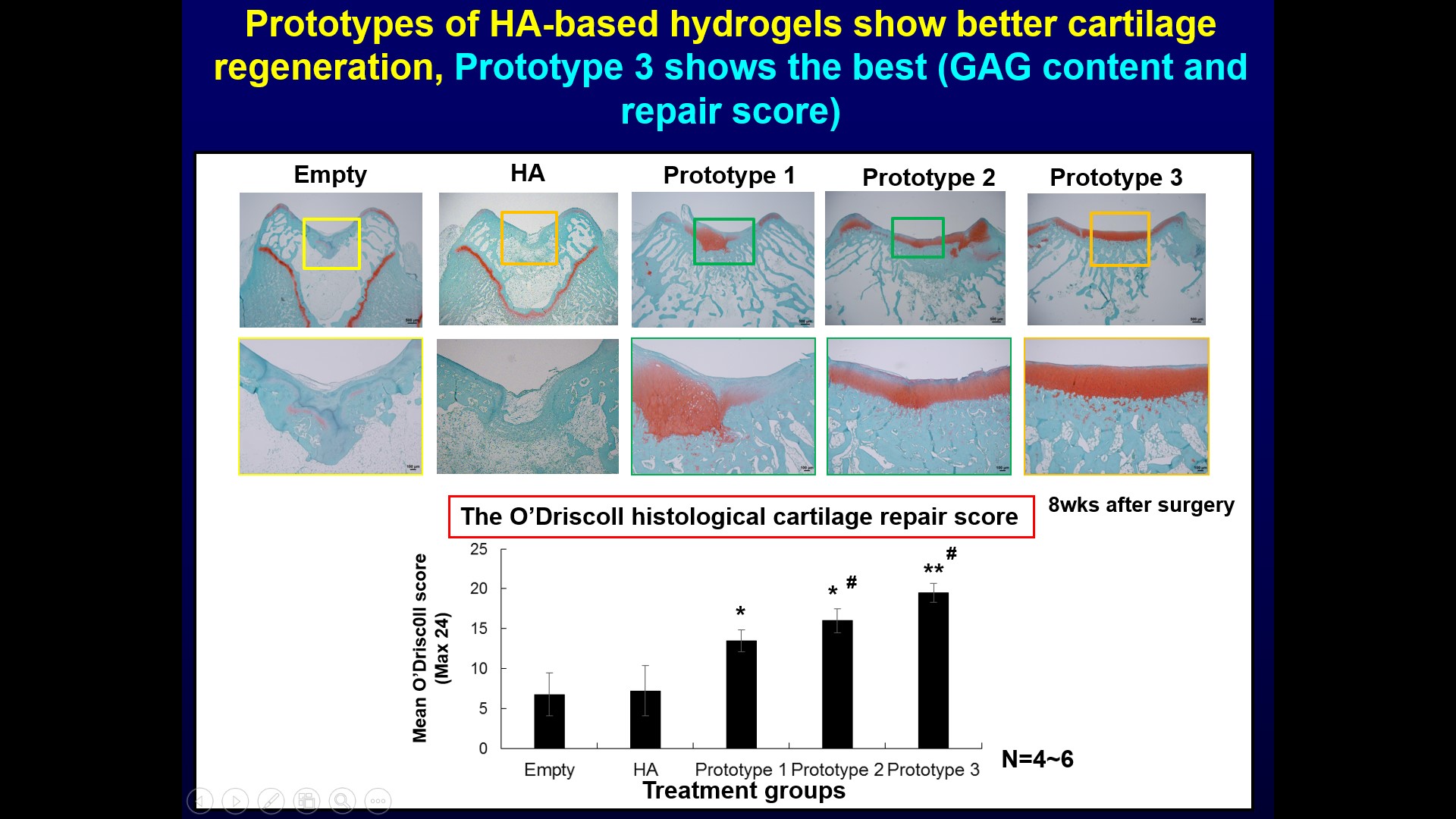
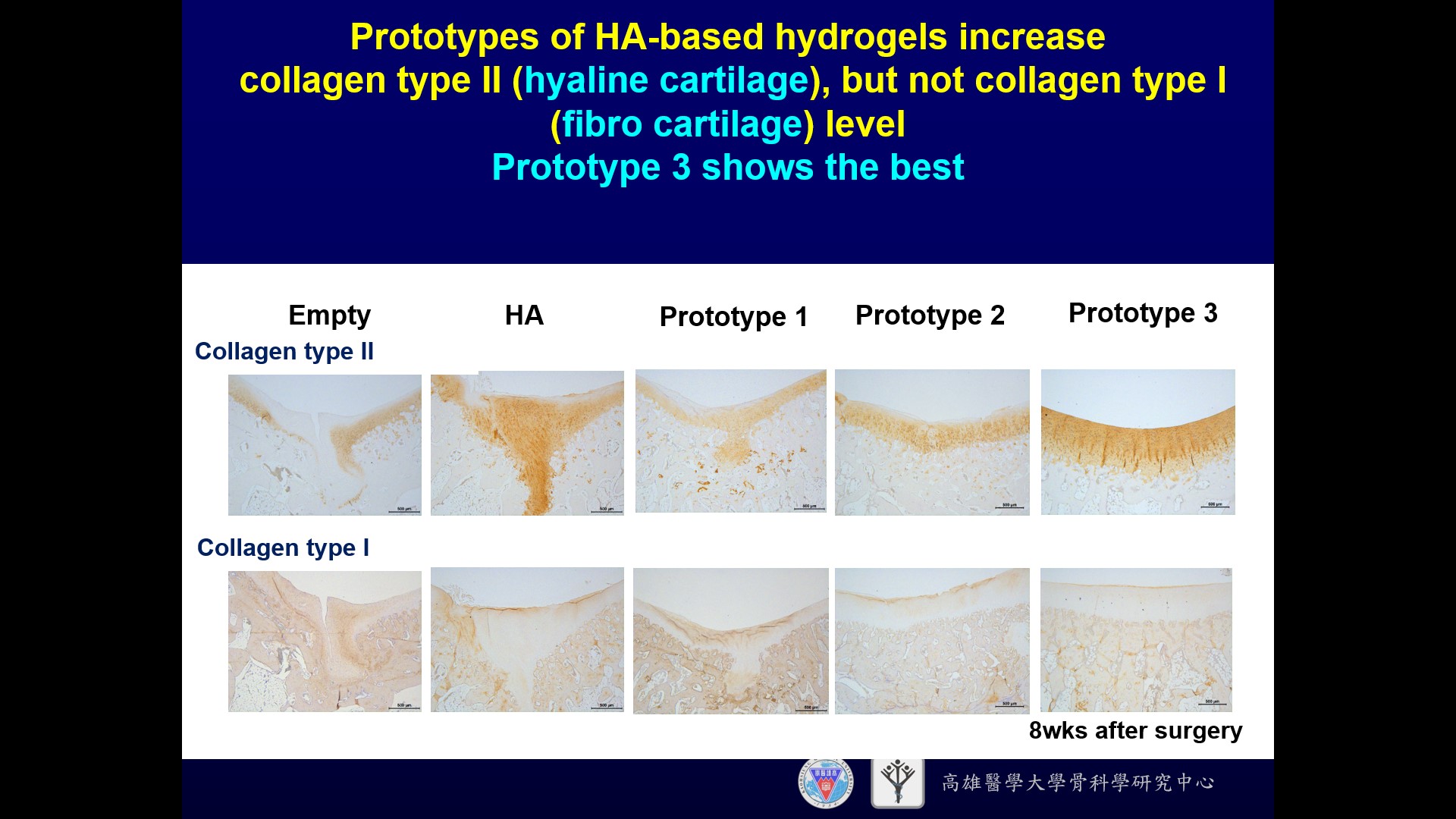
| Summary | The specificity of our product is using hyaluronic acid (HA), clinically used for treating osteoarthritis, as the microenvironment factor to facilitate stem cell chondrogenesis. We further modified the HA hydrogel through optimizing the chemical cue (selecting molecular weight) and physical cue (adjusting stiffness by cross-linkage). The modified novel cross-linked HA hydrogel can promote mesenchymal stem cell chondrogenesis and enhance cartilage tissue formation. The animal study has demonstrated that our HA product can promote the synthesis of hyaline cartilage, but avoid the synthesis of fibrocartilage, by stimulating bone marrow mesenchymal stem cells (BMSCs) in the defect site without additional stem cell implantation. If the defect is larger and more difficult to repair, the HA product can also be implanted with stem cells from any origin, such as adipose derived stem cells, BMSCs, etc. |
||
| Scientific Breakthrough | This technology is a cross-linked hyaluronic acid hydrogel technology, without the need to cooperate with chondrocytes or mesenchymal stem cells. Compared with other clinically used micro-fracture products, the advantage of this technology is that hyaluronic acid is the main component of articular cartilage and Synovial fluid, and has the function of promoting the formation of hyaline cartilage and reducing the formation of fibrous cartilage. Furthermore, hyaluronic acid is the main component of articular cartilage Ingredients, it is less likely to cause immune rejection. |
||
| Industrial Applicability | The target customers of this product are the main markets of medical equipment such as the United States, China, the European Union and Japan. Clinical patients due to articular cartilage defects are diagnosed by orthopedic doctors and used in knee surgery. This technology aims to transfer the technology to the manufacturers that have the production capacity of hyaluronic acid-related medical equipment, including those in the Republic of China, Japan or the United States, to continue production and sales. |
||
| Keyword | hyaluronic acid cross-linked hyaluronic acid stiffness Hyaline cartilage Fibrous cartilage stem cell micro-fracture Non-cell Synovial fluid mesenchymal stem cells | ||
- shunchengwu@hotmail.com
other people also saw