| Technical Name | Novel Therapy for Critical Limb Ischemia | ||
|---|---|---|---|
| Project Operator | National Cheng Kung University | ||
| Project Host | 黃玲惠 | ||
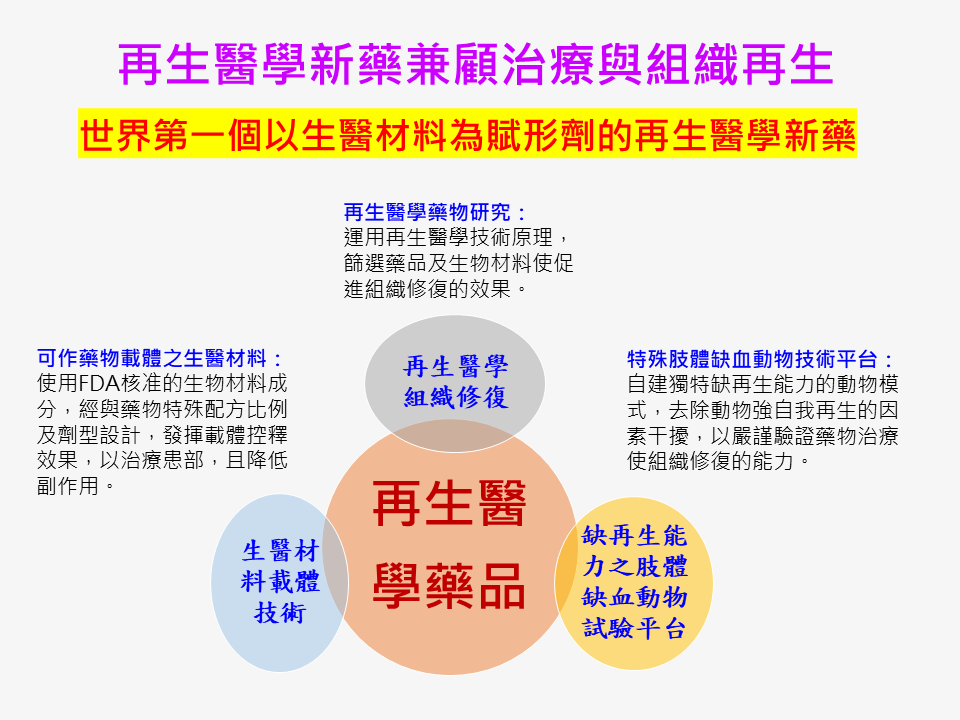
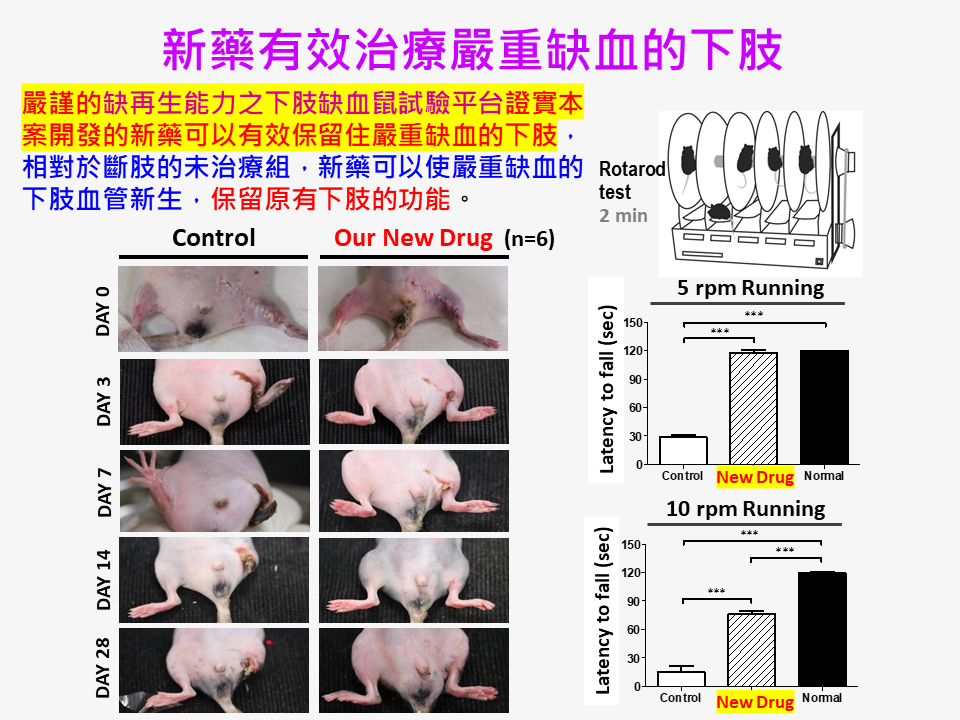
| Summary | We used the concepts of tissue regeneration, embedding drugs in biomaterial carriers,a rigorous regeneration deficient animal model with severe hind limb ischemia to develop an effective novel therapy Grace-001. The new drug can promote neovascularization, tissue regeneration, blood flow maintenance,restoration of blood vessels, nerves,muscles as well as their functions. This new dr |
||
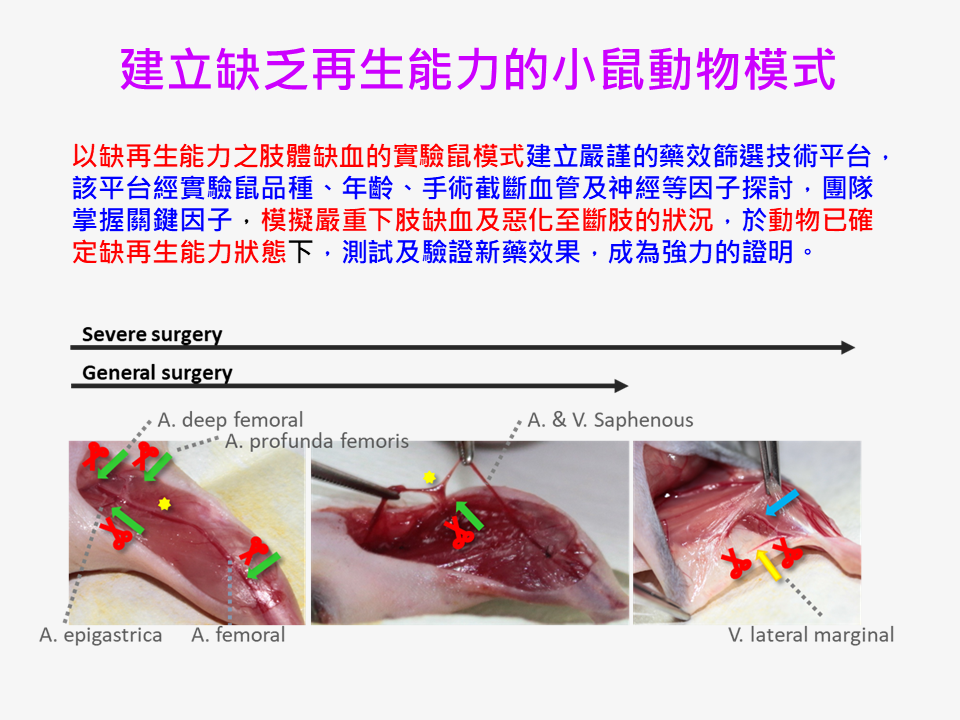
| Scientific Breakthrough | This case is the world's first new concept of novel regenerative medicine. I took the advantage of FDA-approved small molecular drugsbiomaterials, each of which has indicated therapeutic activities, to develop a new specialty drug product Grace-001. Besides, we have established an assessment platform of regeneration-deficient hindlimb-ischemia murine model. We have broken through the bottlene |
||
| Industrial Applicability | The new drug product“Grace-001” fulfills the unmet clinical needs for the critical limb ischemia. As it comprises a series of FDA-approved ingredients, its approval can be achieved under the 505(b)(2) regulatory pathway. In this case, the costs, risktime for marketing will be greatly reduced, whereas the successful ratecompetitive advantages will be increased. Because of that, it saves e |
||
| Matching Needs | - |
||
| Keyword | drug Regenerative medicine critical limb ischemia biomaterial angiogenesis amputation tissue regeneration hindlimb novel therapy muscle regeneration | ||
- lynn@mail.ncku.edu.tw
other people also saw