| Technical Name | A New Hope for the Treatment of Multiple System Atrophy | ||
|---|---|---|---|
| Project Operator | National Taiwan University | ||
| Project Host | 賴文崧 | ||
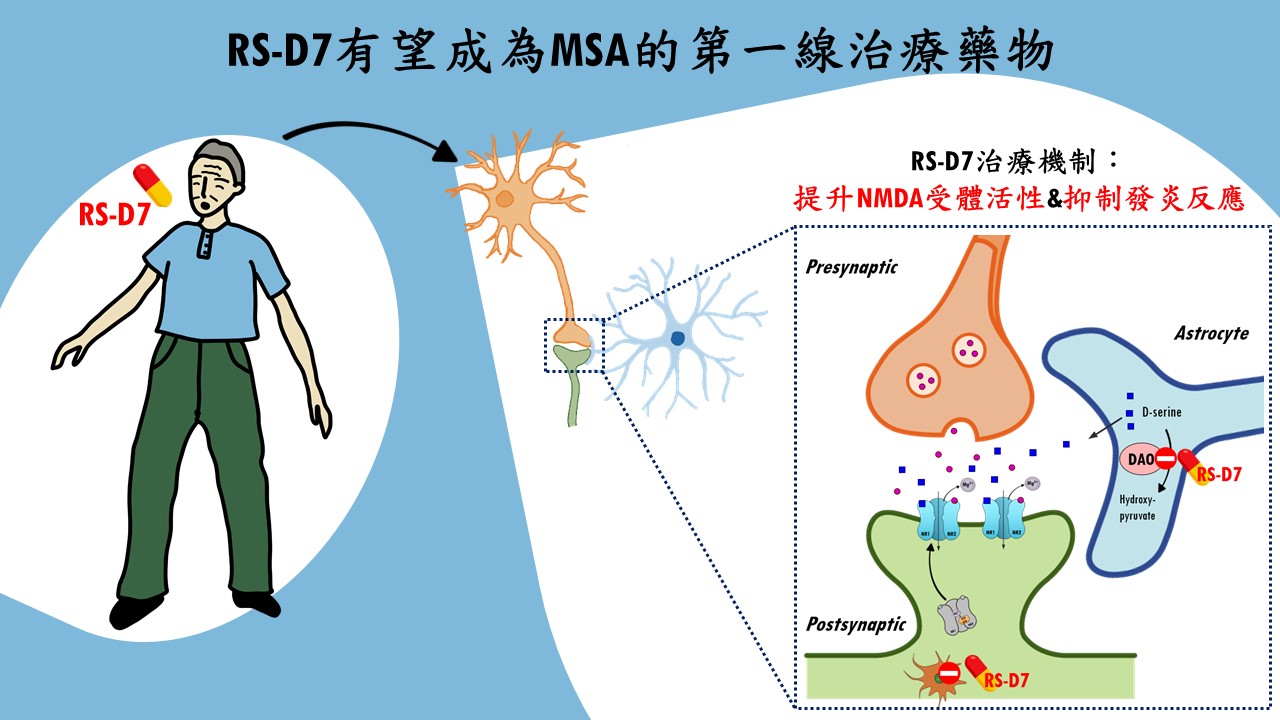
| Summary | "1. The underlying neuropathophysiological mechanisms of MSA remains much unclear. MSA is a severe condition that is seriouswhose treatment is not addressed adequately by current available therapy. 2. RS-D7, a NCEa novel DAO inhibitor, has a great potential to be a first-in-class drug for the treatment of MSA. 3. RS-D7 has a very good safety profileis suitable for long-term use. The therapeutic effect of RS-D7 was confirmed in our preclinical modelsproof-of-concept clinical trial. 4. With intellectual property protectionFDA-approved orphan drug designation (ODD). 5. RS-D7 had been transferred to Yoda Pharmaceutical Inc.the preparations of FDA pre-INDIND application are in progress." |
||
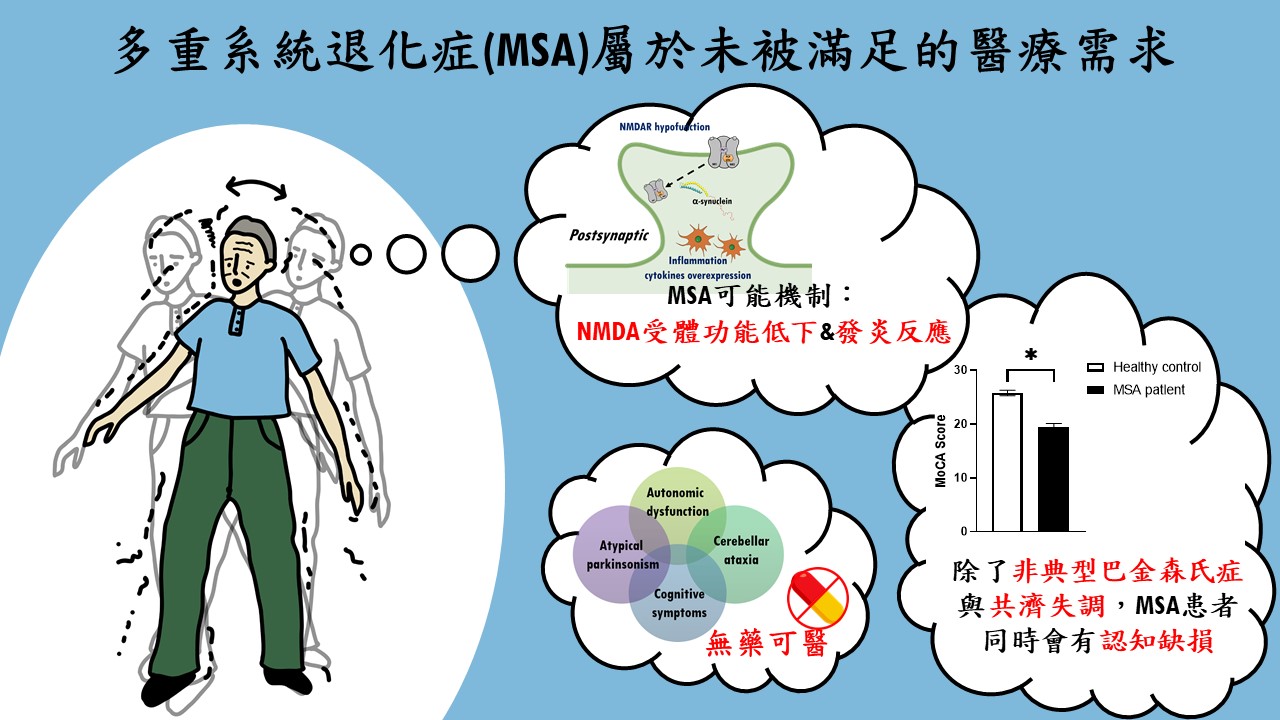
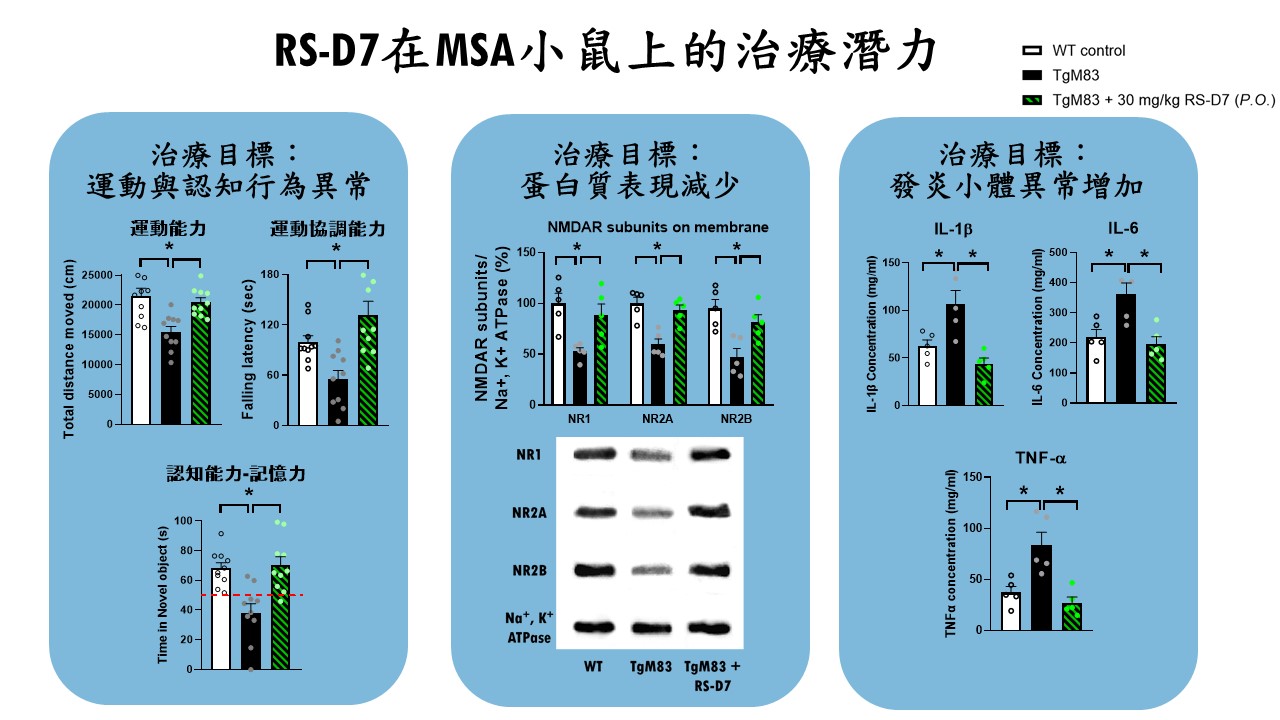
| Scientific Breakthrough | MSA is an urgent unmet medical need with ataxiacognitive impairments. Breaking with tradition, our research team aims at developing a novel DAO inhibitorexploring new indications for RS-D7. Taking advantage of preclinical animal models with behavioral phenotypinginvestigation of neural mechanism, TgM83 mice with synucleinopathies were applied to model MSA-related motorcognitive deficits. Our findings demonstrate that RS-D7 ameliorates these observed behavioral deficitsenhances the expression of membrane-bound NMDA receptors as well as inhibition of inflammation cytokines. These results support the involvement of NMDARs in the pathogenesis of MSAtherapeutic potential of RS-D7 in the treatment of MSA. |
||
| Industrial Applicability | The estimated market value for the treatment of MSA is over $150 million. However, currently existing drugs show no therapeutic effect for MSA. Our preclinical results indicate therapeutic effect of RS-D7, a NCEa novel DAO inhibitor, in the alleviation of MSA-related motor deficitscognitive impairments. Our research team has received several awards for new drug development, including National Innovator Award in 20162019,Future Tech Exhibition of MOST in 20172019. Our intellectual property protection for RS-D7 is nearly completed. FDA orphan drug designations for RS-D7 was approved lately for the treatment of MSA. The IP rights for RS-D7 had been transferred to Yoda Pharmaceutical Inc. for further development. |
||
| Keyword | multiple system atrophy (MSA) ataxia cognitive impairments new drug development NMDA receptor DAO inhibitor orphan drug unmet medical need patents | ||
- Contact
- Da-Zhong Luo
- D06227102@ntu.edu.tw
other people also saw