| Technical Name | 泌尿道上皮癌微型 RNA 篩檢套組 | ||
|---|---|---|---|
| Project Operator | National Central University | ||
| Project Host | 馬念涵 | ||
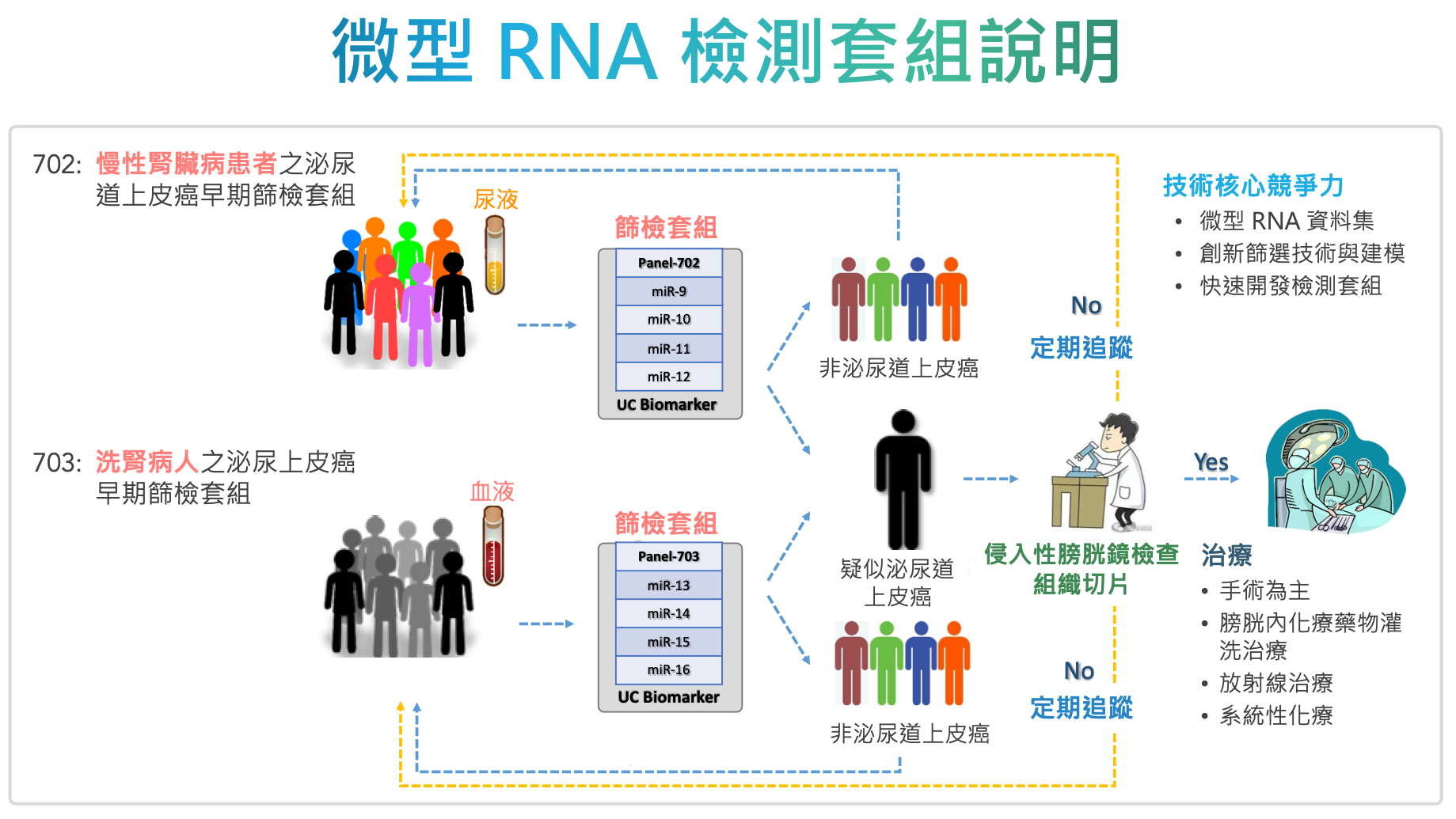
| Summary | The screening kits were developed using miRNAs for high-risk populations for urothelial carcinoma (UC). Products 702703 are designed for chronic kidney diseasehemodialysis patients using urineblood samples, respectively. With high accuracyminimal invasiveness, the kits are intended to be used as a screeningregular monitoring of UC. If a test reveals a positive result, the patient then undergoes cystoscopic examination for further confirmation. Otherwise, the patent only needs to be monitored annually. |
||
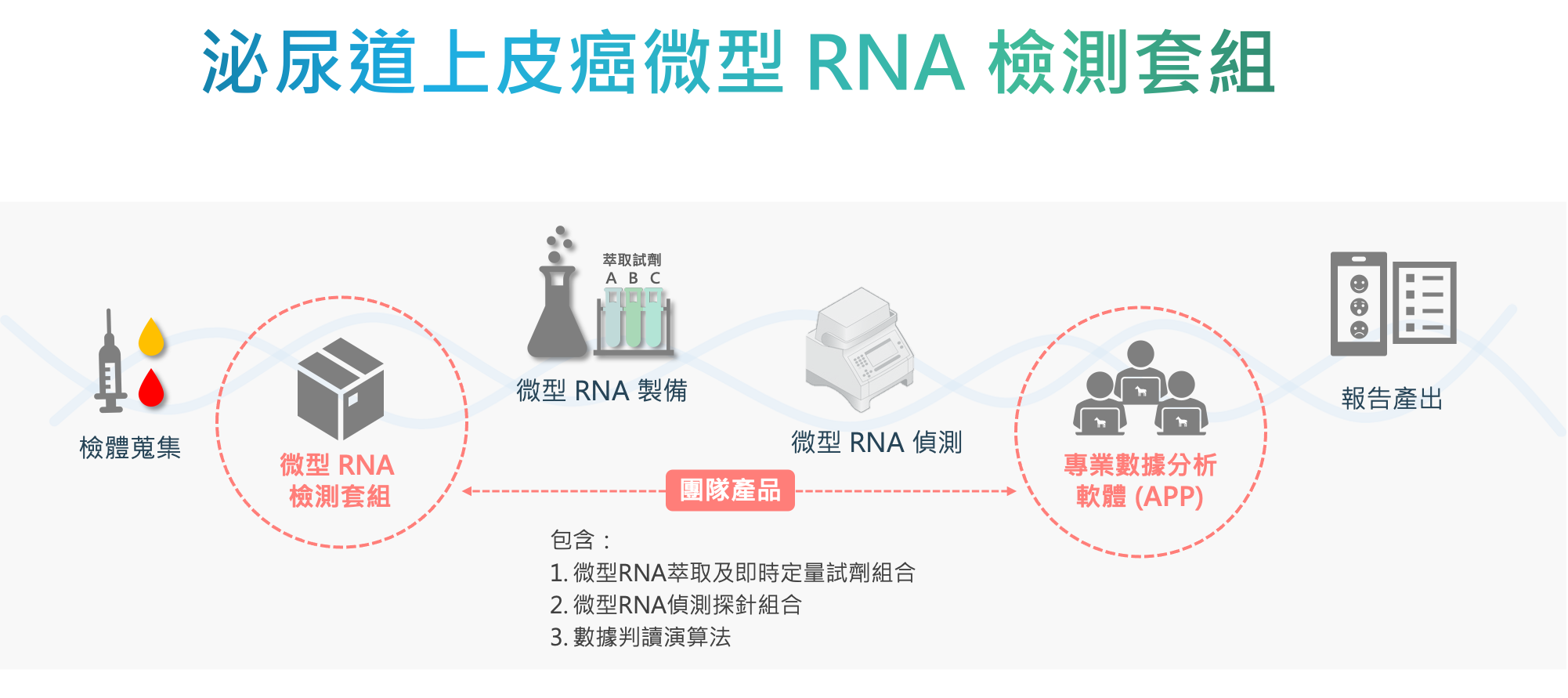
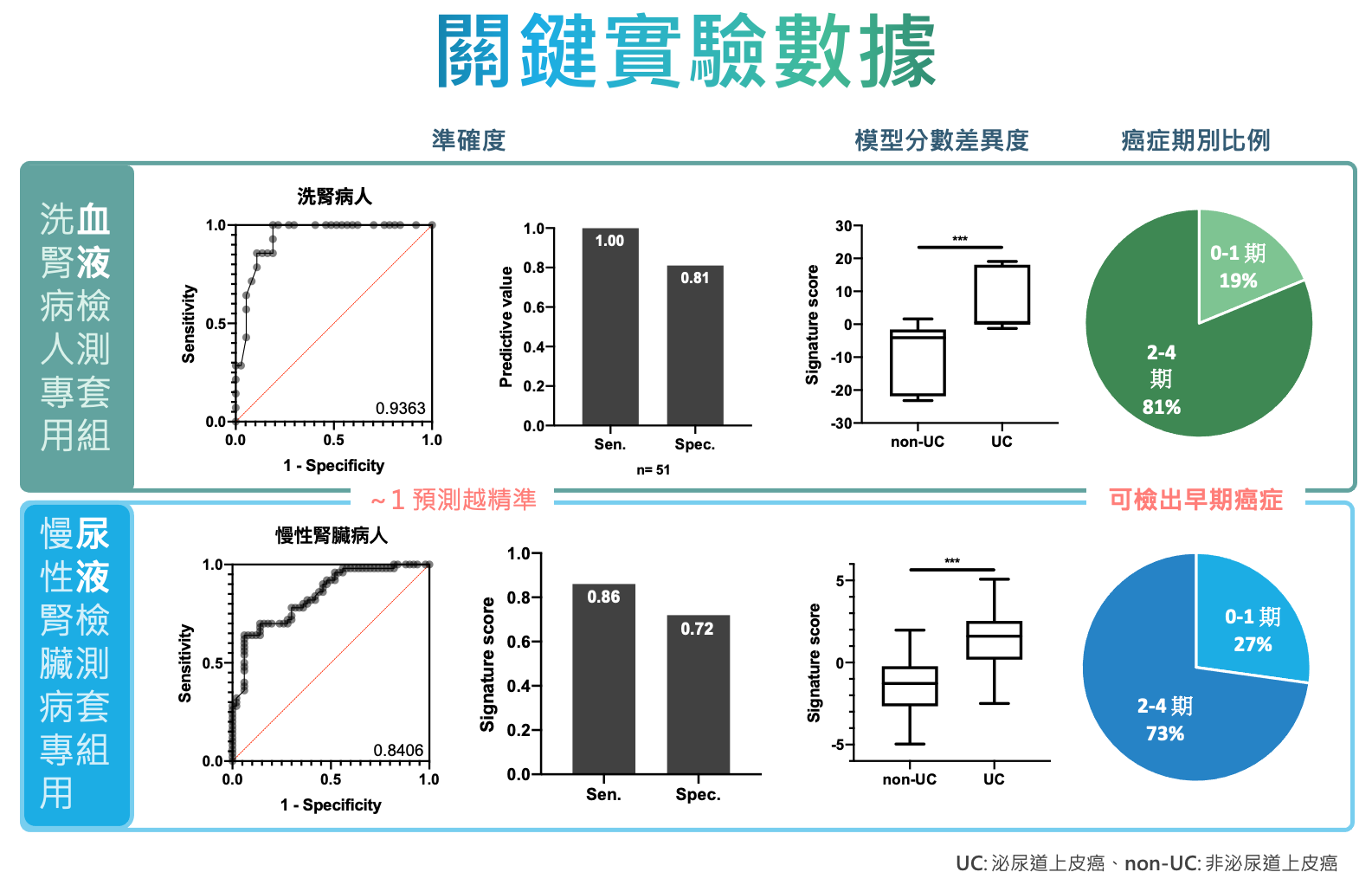
| Scientific Breakthrough | We selected miRNAs that are highly specific to urothelial carcinoma (UC) tissues as biomarkers to develop 2 screening kits for patients with chronic kidney diseasehemodialysis patients. miRNA expressions are analyzed by commercially available qRT-PCR machinesinterpreted along with biochemistries by our proprietary algorithms. With a novel RNA extraction methodproper normalization, our kit provides stable detection of trace molecules in body fluids with high sensitivity, high specificity,minimal invasiveness. |
||
| Industrial Applicability | Our urothelial carcinoma miRNA screening kits are developed for high-risk populations. Without affecting clinical procedures in screeningmonitoring urothelial carcinoma, these kits provide screening tools with minimal invasiveness, high sensitivity,patient welfare. Current screening tests have limitationsresult in discomfort. Our kits increase patient’s willingness to be tested by minimizing test-related riskspressure. The cancer cure ratesurvival therefore can be improved,the loading of health professionalsthe health systems can also be relieved. |
||
| Matching Needs | 天使投資人、策略合作夥伴 |
||
| Keyword | microRNA (miRNA) Urothelial carcinoma (UC) Chronic kidney disease (CKD) Hemodialysis (HD) Disease biomarker Screening kit Molecular diagnostics RT Q-PCR Precision medicine miRNA tests development platform | ||
- charlie850729@gmail.com
other people also saw