| Technical Name | Establishment of oral carnitine challenge test for microbiota-directed personalized nutrition | ||
|---|---|---|---|
| Project Operator | National Taiwan University Hospital, Department of Internal Medicine | ||
| Project Host | 吳明賢 | ||
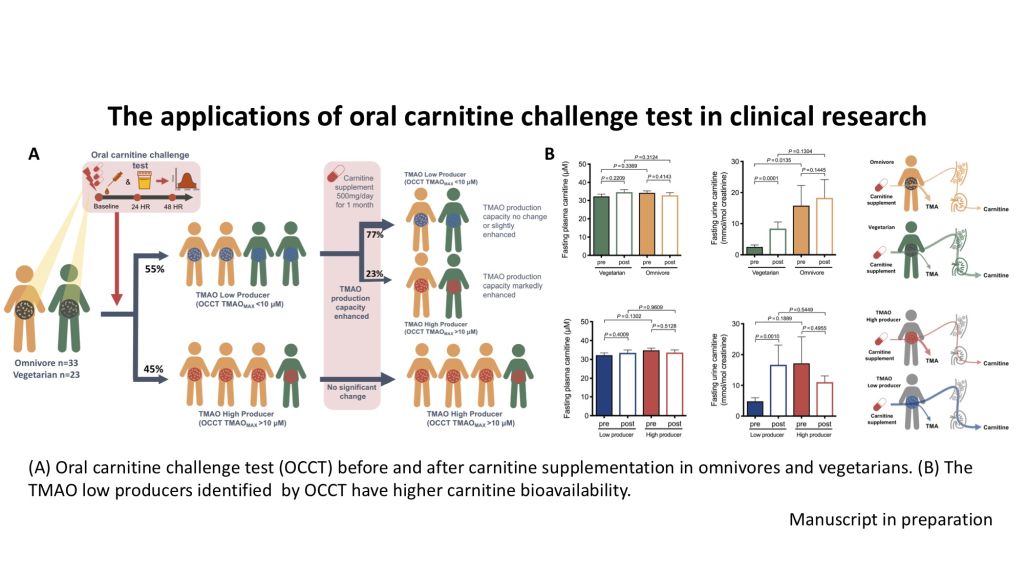
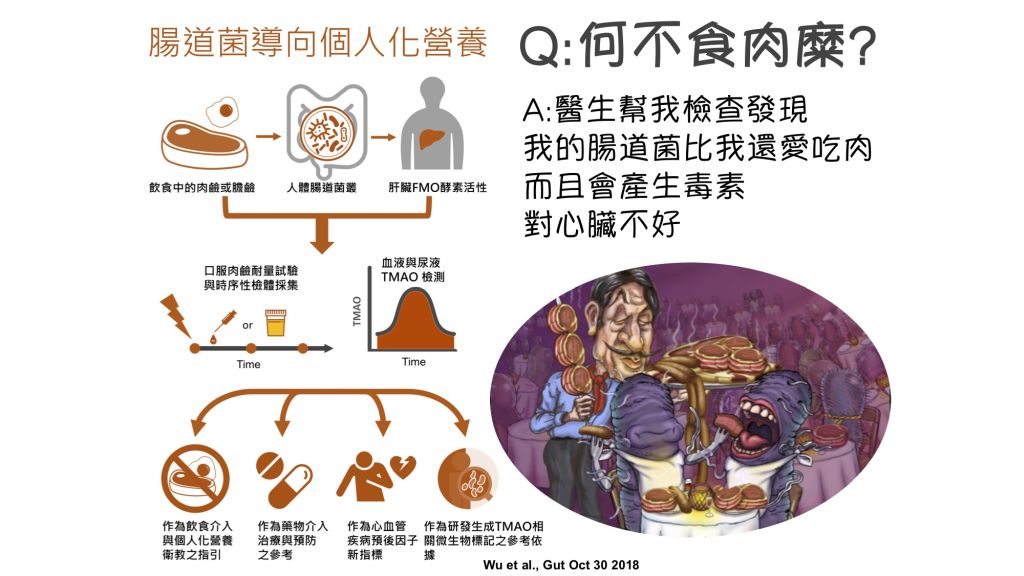
| Summary | The OCCT can serve as a clinical gut microbiota functional test to effectively measure the TMAO producing capacity from gut microbiota. Because chronic exposure to TMAO has been proved to promote atherosclerosis and thrombosis, the result of OCCT could serve as a microbiota-directed personalized nutritional guidance to help improve or prevent the development of cardiovascular disease. |
||
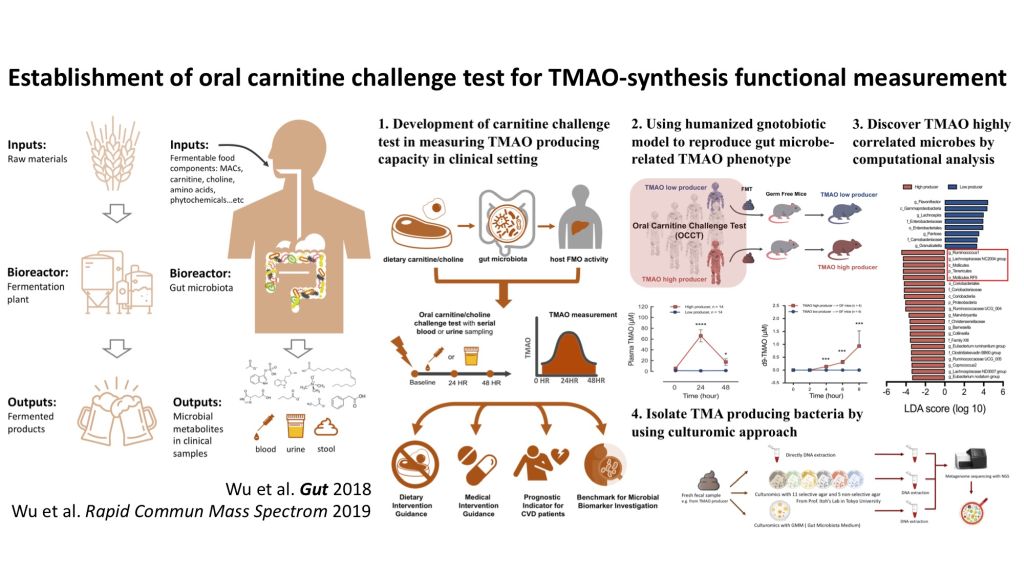
| Scientific Breakthrough | The design of challenging dose of carnitine and sampling times in our OCCT is based on a strict pharmacokinetics study. So that we can load non-isotope labeled carnitine to produce a peak area for TMAO producing capacity calculation. Besides, we found the urine TMAO is highly correlated to plasma TMAO, which means we can use urine to replace plasma to increase the feasibility and convenience of this test. |
||
| Industrial Applicability | The OCCT developed in our study can become a clinical gut microbiota functional test. For the aspect of precision medicine, the test can provide scientific evidence in controlling meat consumption in patients with cardiovascular disease. The test could also become an item in health examination to serve as a personalized guidance for dietary education. The OCCT can be even more competitive in market if it can be integrated into a convenient kit. |
||
| Keyword | Oral carnitine change test Gut microbiota functional test Trimethylamine N-oxide Cardiovascular disease Atherosclerosis Gut microbial metabolite Gut microbiota Personalized nutrition Precision medicine Preventive medicine | ||
- weikaiwu0115@gmail.com
other people also saw