| Technical Name | Fabrication of coronavirus protein microarray for blood diagnosis and reagent development | ||
|---|---|---|---|
| Project Operator | National cheng kung university | ||
| Project Host | 許觀達 | ||
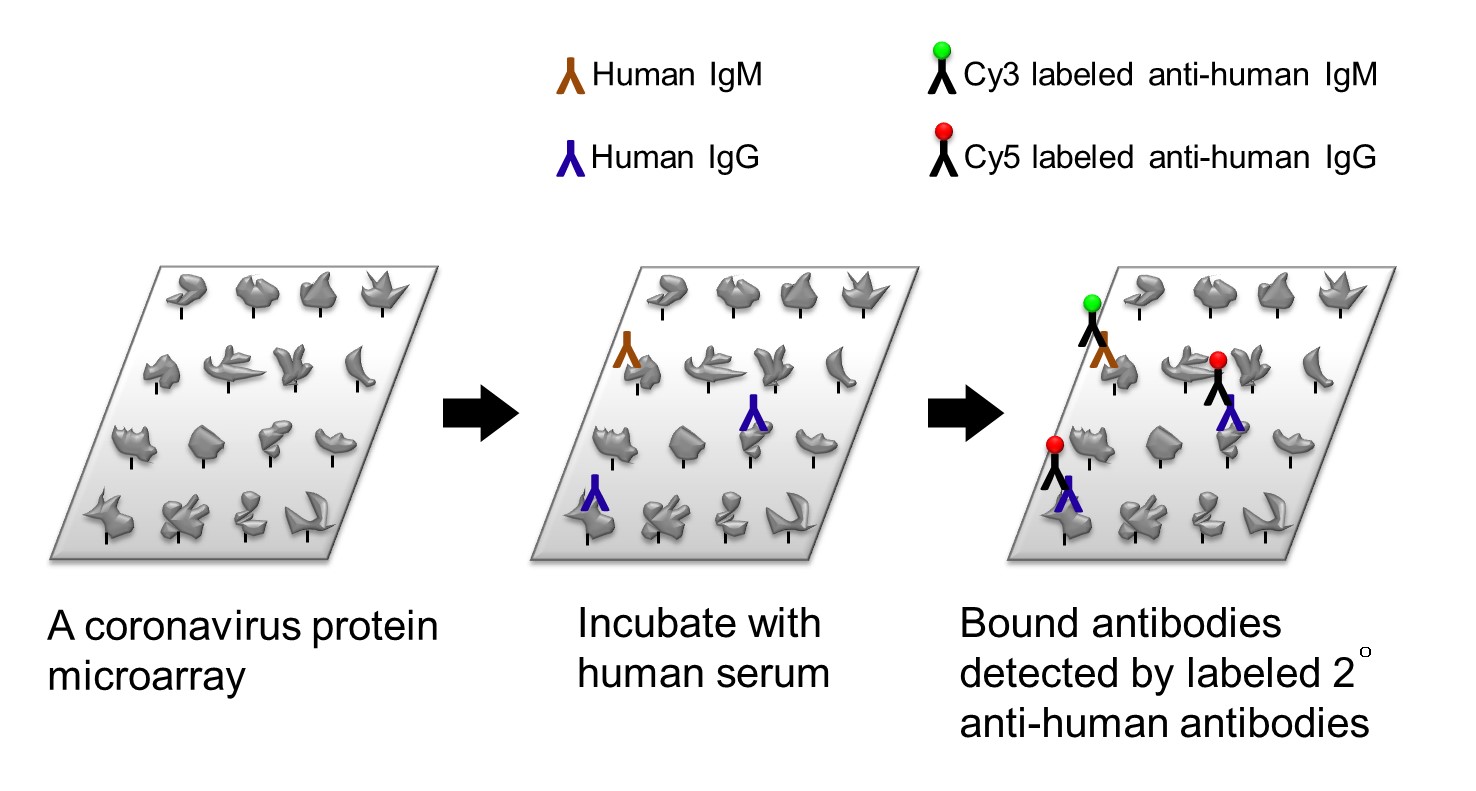
| Summary | Current detection of SARS-CoV-2 is based on the real-time RT PCR on the respiratory specimens which could be limited by local viral load. Analyzing the humoral immune responses against coronavirus antigens is one of the complementary methods to identify the SARS-CoV-2 infection because humoral immune responses last for a long period of time. Here we fabricated coronavirus protein microarrays which included proteins from SARS-CoV, MERS-CoV, and SARS-CoV-2. We showed the high sensitivity of this protein array with detection limits lower than 50 pg. By profiling the serum IgG and IgM from 30 COVID-19 patients and 30 controls, the array demonstrated 97% sensitivity and 97% specificity which is outperformed than the requirement by FDA. Moreover, we discovered a set of biomarkers for the COVID-19 diagnosis which can be translated to rapid test. Currently the coronavirus protein array is under massive production. We welcome licensing or collaboration if you are interested in our technology. |
||
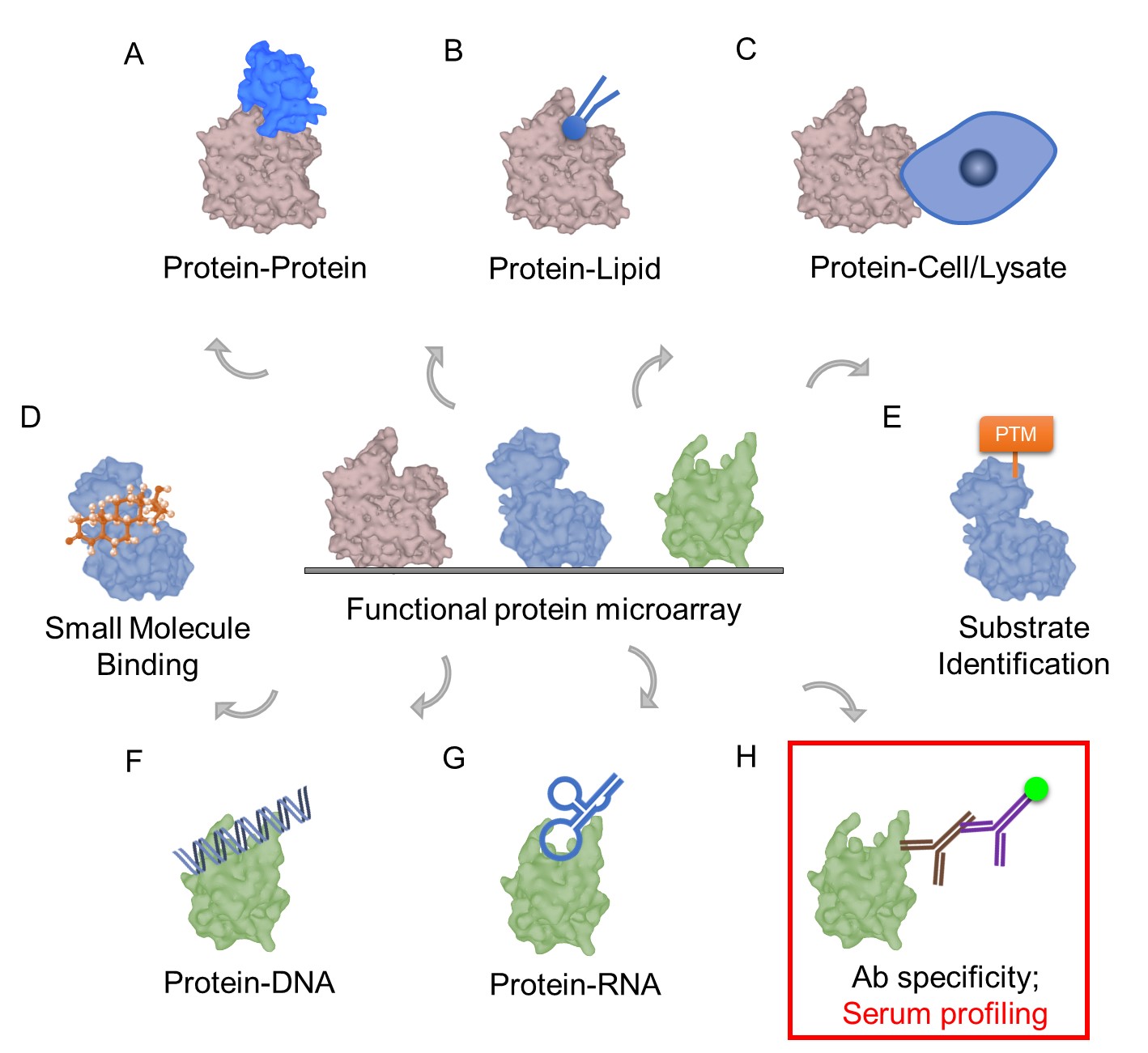
| Scientific Breakthrough | Multiplexed. Simultaneously detect serum IgG/IgM and profile the immune responses against many coronavirus proteins. |
||
| Industrial Applicability | The most wildly used method for COVID-19 diagnosis is detecting the nucleic acid of SARS-CoV-2 RNA in the nasal swab. However, the nasal swab is uncomfortable to the subjects, increases the risk of transmission to the medical practitioners, and needs multiple samplings for the diagnosis. In contrast, serological testing provides an easier solution for both subjects and medical practitioners. To date, there are several rapid tests based on serological antibody against N or S proteins with low sensitivity 45~95%. Since humoral responses varies from person to person and react to different viral compartments with variety specificity. Our protein microarray is the best solution for the task which includes proteins from many coronaviruses and shows the highest 97% sensitivity for the diagnosis. |
||
| Keyword | COVID-19 protein microarray coronavirus SARS-CoV-2 antibody diagnosis rapid test antigen, immune blood | ||
- guanda@gs.ncku.edu.tw
other people also saw