| Technical Name | A vaccine kit against multiple dengue virus serotypes and preparation and application thereof | ||
|---|---|---|---|
| Project Operator | National Tsing Hua University | ||
| Project Host | 吳夙欽 | ||
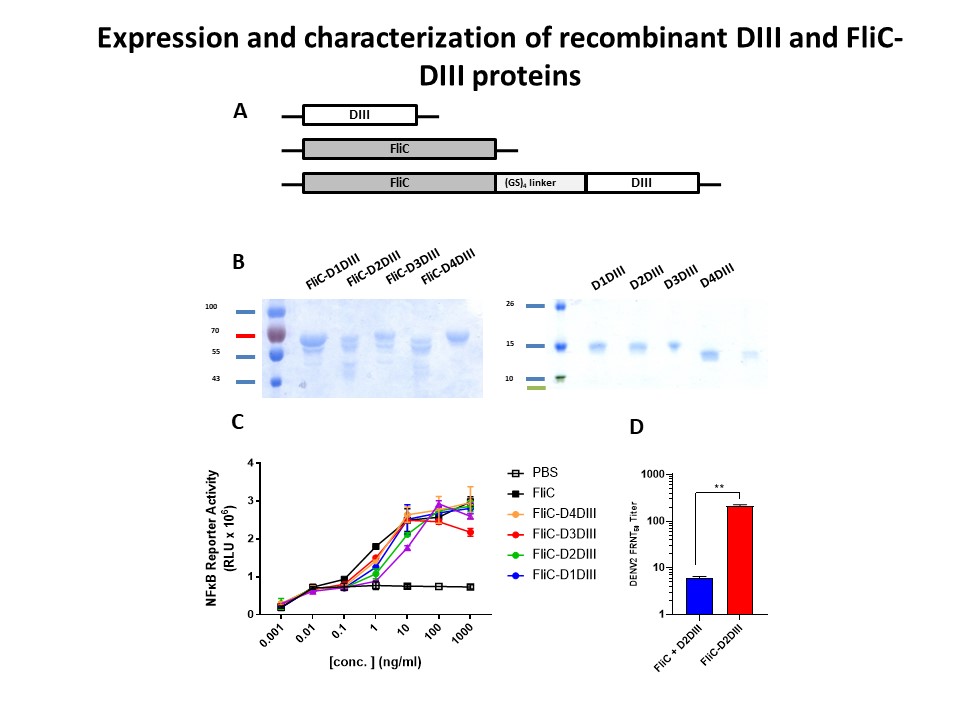
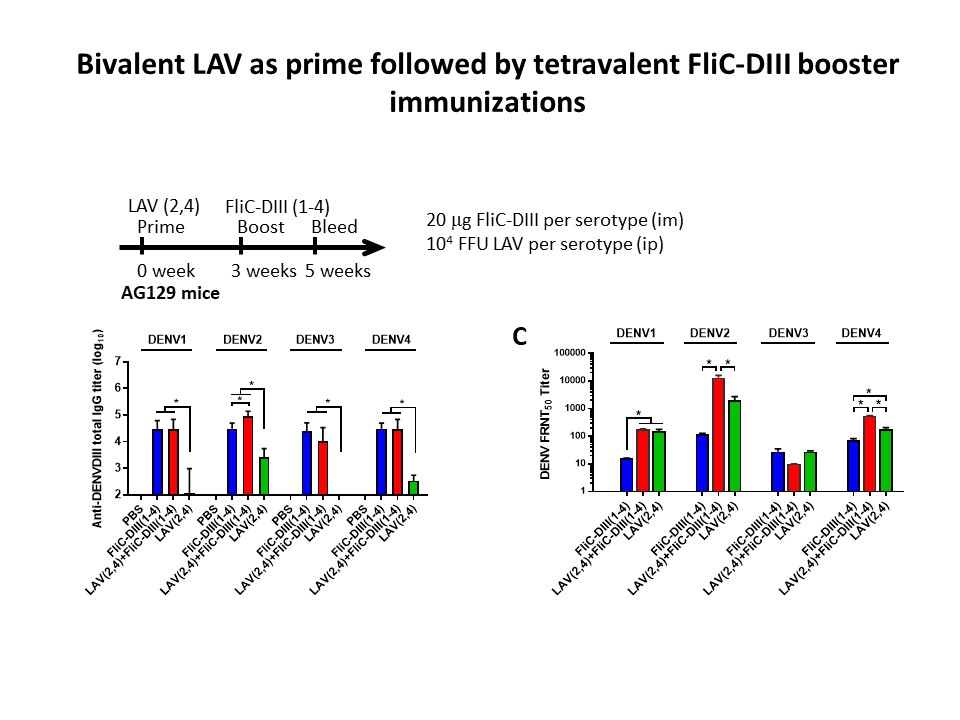
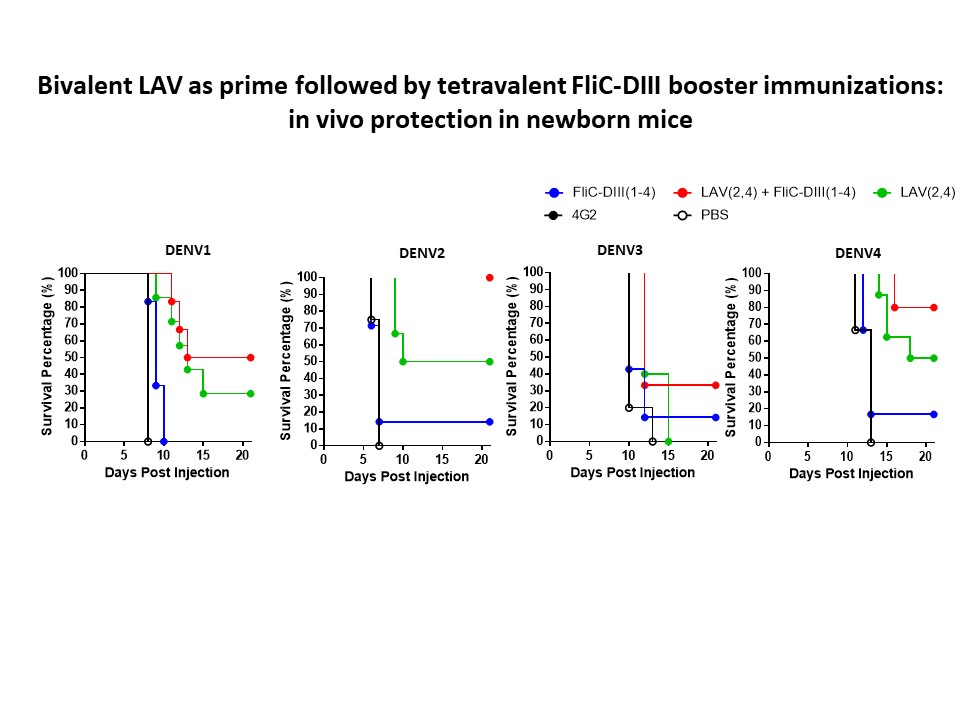
| Summary | The present invention provides a vaccine combination against multiple dengue virus serotypes, including a first vaccine and a second vaccine, wherein the first vaccine includes a live-attenuated dengue virus and a live-attenuated chimeric dengue virus, and the second vaccine includes a plurality type of recombinant flagellin and envelope domain III fusion proteins, wherein an envelope domain III of each type of the recombinant flagellin and envelope domain III fusion proteins is derived from a different dengue virus serotype, and wherein the vaccine combination provides protection against more dengue viruses serotypes than the live-attenuated dengue viruses in the first vaccine. |
||
| Scientific Breakthrough | A vaccine combination against multiple DENV serotypes, comprising a first vaccine and a second vaccine, wherein the first vaccine comprises live-attenuated DENVs, and the second vaccine comprises a plurality type of recombinant flagellin and DIII fusion proteins, and wherein the vaccine combination provides protection against more DENV serotypes than the live-attenuated DENV in the first vaccine. |
||
| Industrial Applicability | The prime-boost immunization with the first priming with the tetravalent live-attenuated DENV vaccines that have been developed and licensed by Sanofi, and the phase III-stage tetravalent live-attenuated Takeda and US NIH vaccines. This invention provides the second dose boosting with tetravalent flagellin-DIII fusion antigen for dengue vaccine development. |
||
| Keyword | vaccine dengue virus four serotypes envelope protein domain III bacterial flagellin fusion protein prime-boost neutralizing antibody immunization kit | ||
- scwu@life.nthu.edu.tw
other people also saw