| Technical Name | TMD1 protein for treating bone loss diseases | ||
|---|---|---|---|
| Project Operator | Kaohsiung medical university | ||
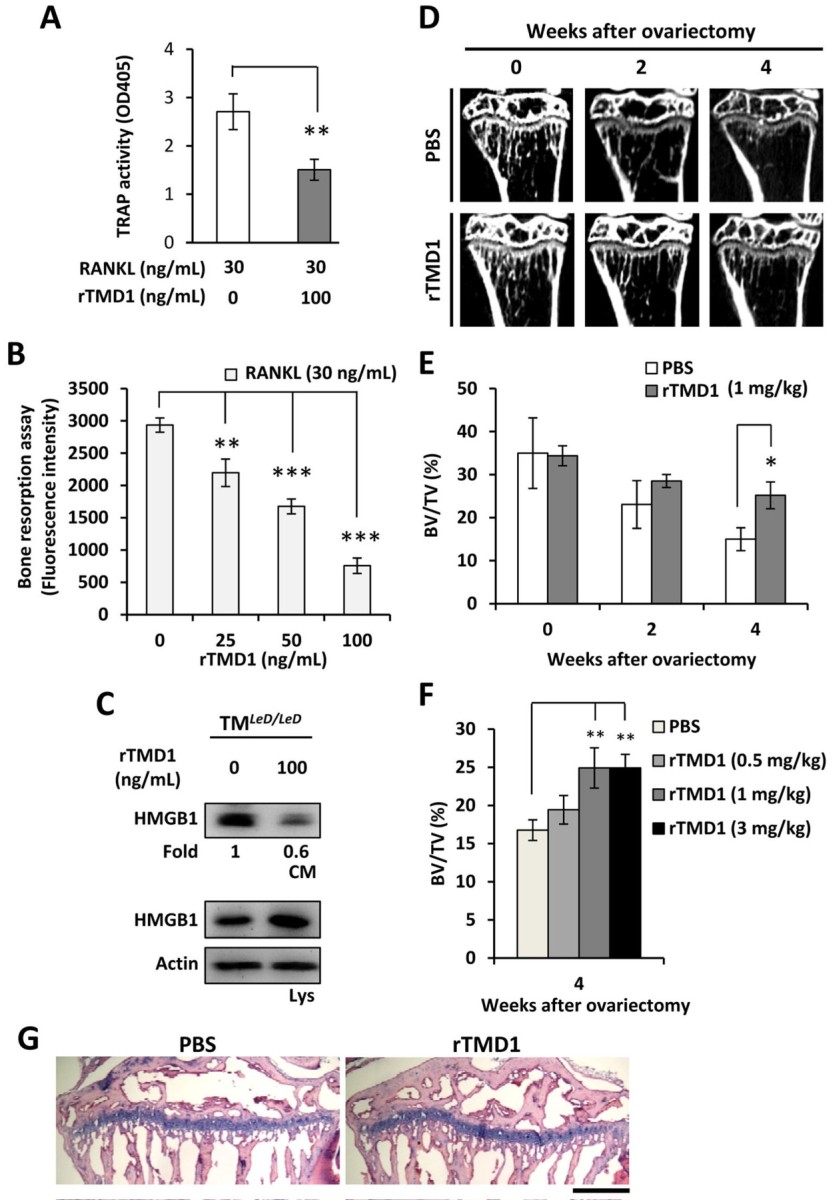
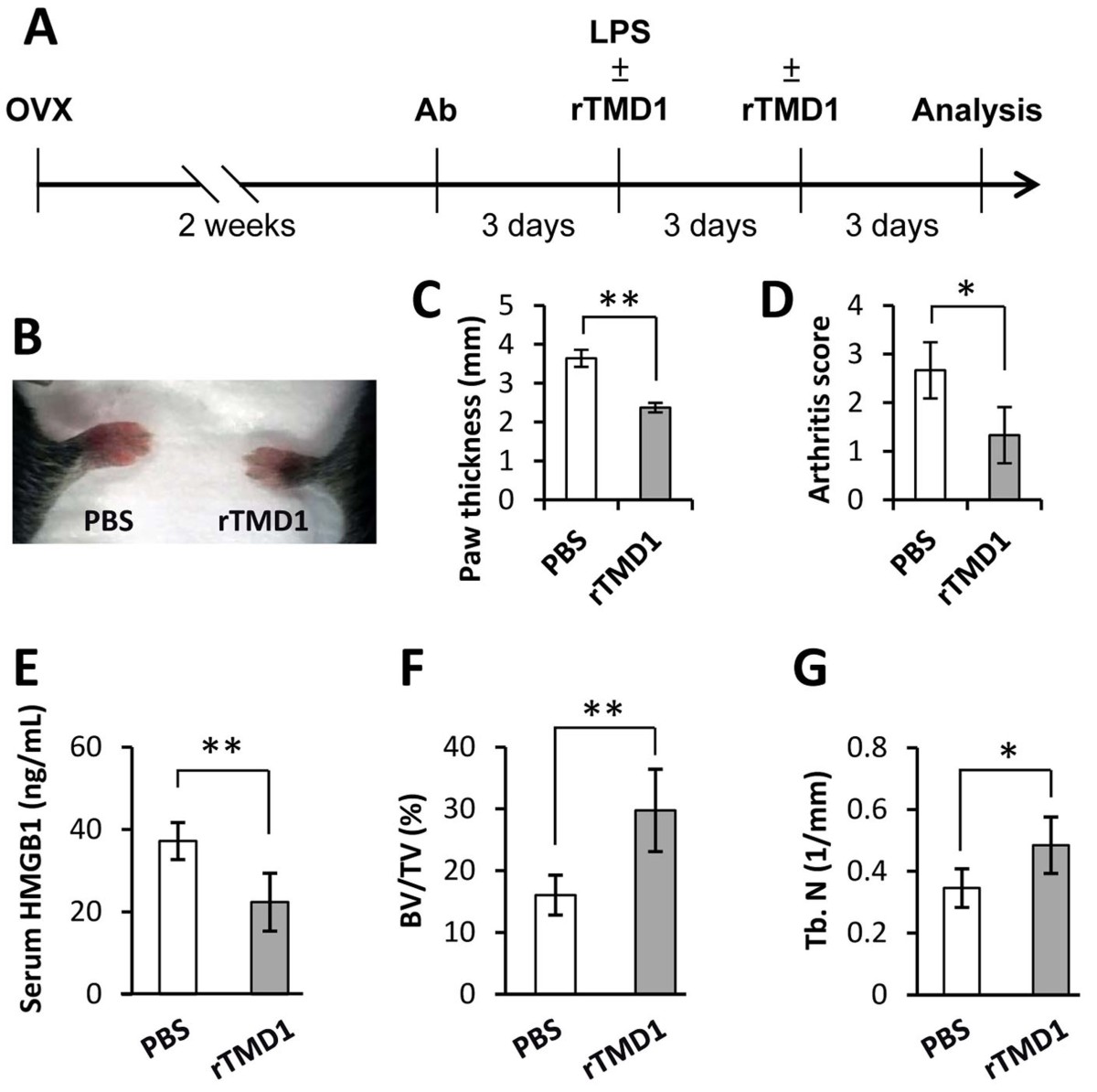
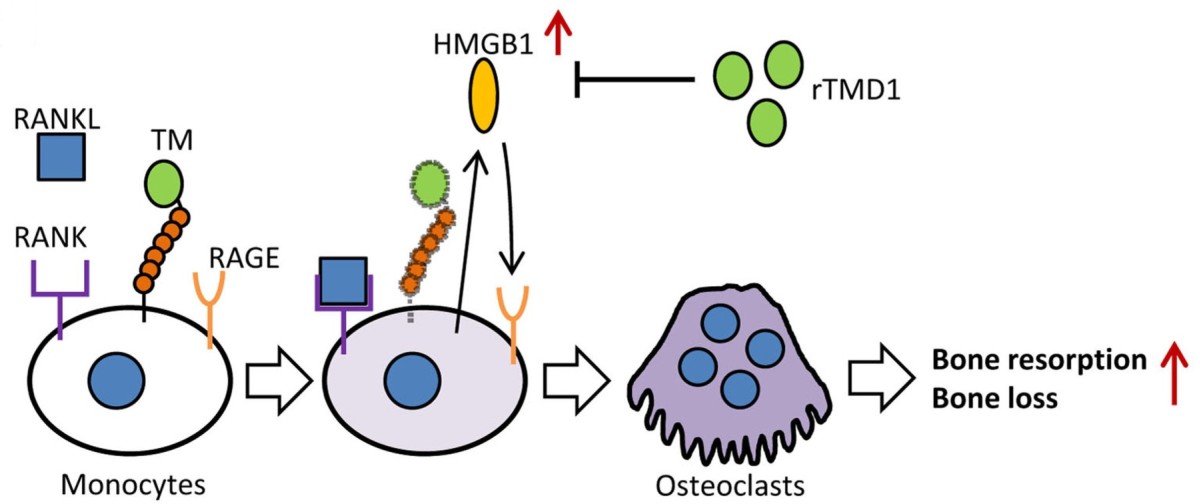
| Summary | The growing evidences indicated that excessive inflammation promotes the formation of osteoclasts, and then causes the bone loss and related disorders such as osteoporosis. Receptor activator of nuclear factor-κB ligand (RANKL) is a critical factor to induce the differentiation of osteoclasts from bone marrow-derived macrophages (BMM). The mechanism of RANKL is promotes the production of extracellular high mobility group box-1 (HMGB1) of BMM, and which activate the receptor for advanced glycation end products (RAGE) on the cell surface, finally finish the differentiation of osteoclasts. On the other hand, thrombomodulin lectin-like domain (TMD1) has been demonstrated that can inhibit the inflammation by interfere the interaction of HMGB1 and RAGE. However, the physiological functions and possible application of thrombomodulin in bone metabolism are unclear. Using the model of RANKL-induced osteoclast differentiation in RAW264.7 cells and tissue-specific gene manipulation technology, we showed for the first evidences that TMD1 function as negative mediator for osteoclastogenesis, and deletion of TMD1 in macrophages leading to an enhancement on the formation of osteoclasts. Furthermore, our animal studies showed that treatment of TMD1 also significantly attenuates the bone loss in both ovariectomized mice and collagen antibody-induced arthritis mice. Thus, we indicate that TMD1 should be a new therapeutic treatment for bone loss diseases. |
||
| Scientific Breakthrough | 本案的目的是在於治療骨質流失之疾病,因目前人們會因生理因素或其他外在因素而有骨質的流失或骨密度的下降的問題,會導致骨質疏鬆症,其會有骨折、腰酸背痛、行動能力受限,甚至無法行動等現象發生。一般來說,人體的骨骼會不斷更新,以維持強韌而優良的品質,而年輕時「造骨」速度高於「蝕骨」速度,因此骨質總量及骨質密度得以持續上昇;而到35歲左右,骨質密度達到顛峰,稱之為「尖峰骨本」。但伴隨年齡增長,骨質流失的速度要高於骨質製造的速度,於是骨質密度逐漸下降。其中,蝕骨細胞是造成骨質流失的主因之一,而本案正是透過抑制Receptor activator of nuclear factor-κB ligand (RANKL)所誘導的蝕骨細胞生成,以達到治療骨質疏鬆症之效果。 |
||
| Industrial Applicability | 全球老年人口逐漸增加,因此骨質流失相關病變的預防與治療將是一個相當無法忽視的課題。 |
||
other people also saw