| 技術名稱 | 武裝型T細胞之創新癌症療法技術 | ||
|---|---|---|---|
| 計畫單位 | 臺北醫學大學 | ||
| 計畫主持人 | 莊國祥 | ||
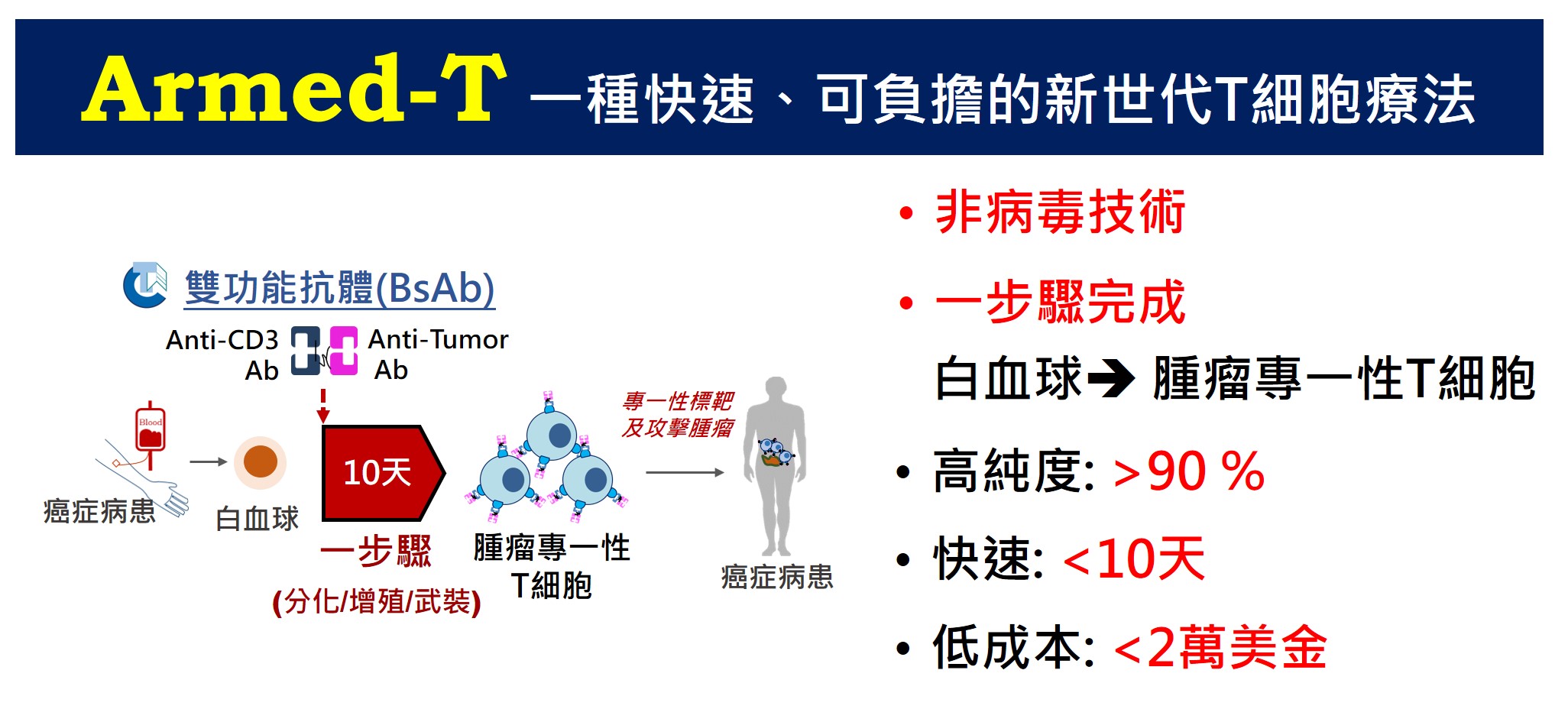
| 技術簡介 | 本團隊開發出一套創新的雙功能抗體(BsAb)培育腫瘤專一性T細胞之技術平台:利用特殊構型之anti-cancer/anti-CD3 BsAb與人類周邊血液單核球共同培養,即可於10天內,一步驟大量增殖各式腫瘤專一性T細胞(武裝型T細胞 / BsAb-armed T,純度>90%);是一套快速培育、非病毒基因轉殖、高純度且成本合宜之腫瘤專一性T細胞建置平台。 |
||
| 科學突破性 | 臨床CAR-T細胞療法仍存在諸多問題(反轉錄病毒技術、純度僅37%、30天製備、要價1500萬元);武裝型T細胞療法使用特殊雙功能抗體(非病毒基因技術)與病患白血球共培養,僅需10天即可培育出腫瘤專一性T細胞(純度>90%),大幅降低製備成本,讓更多病患能獲得腫瘤專一性T細胞療法以拯救生命。 |
||
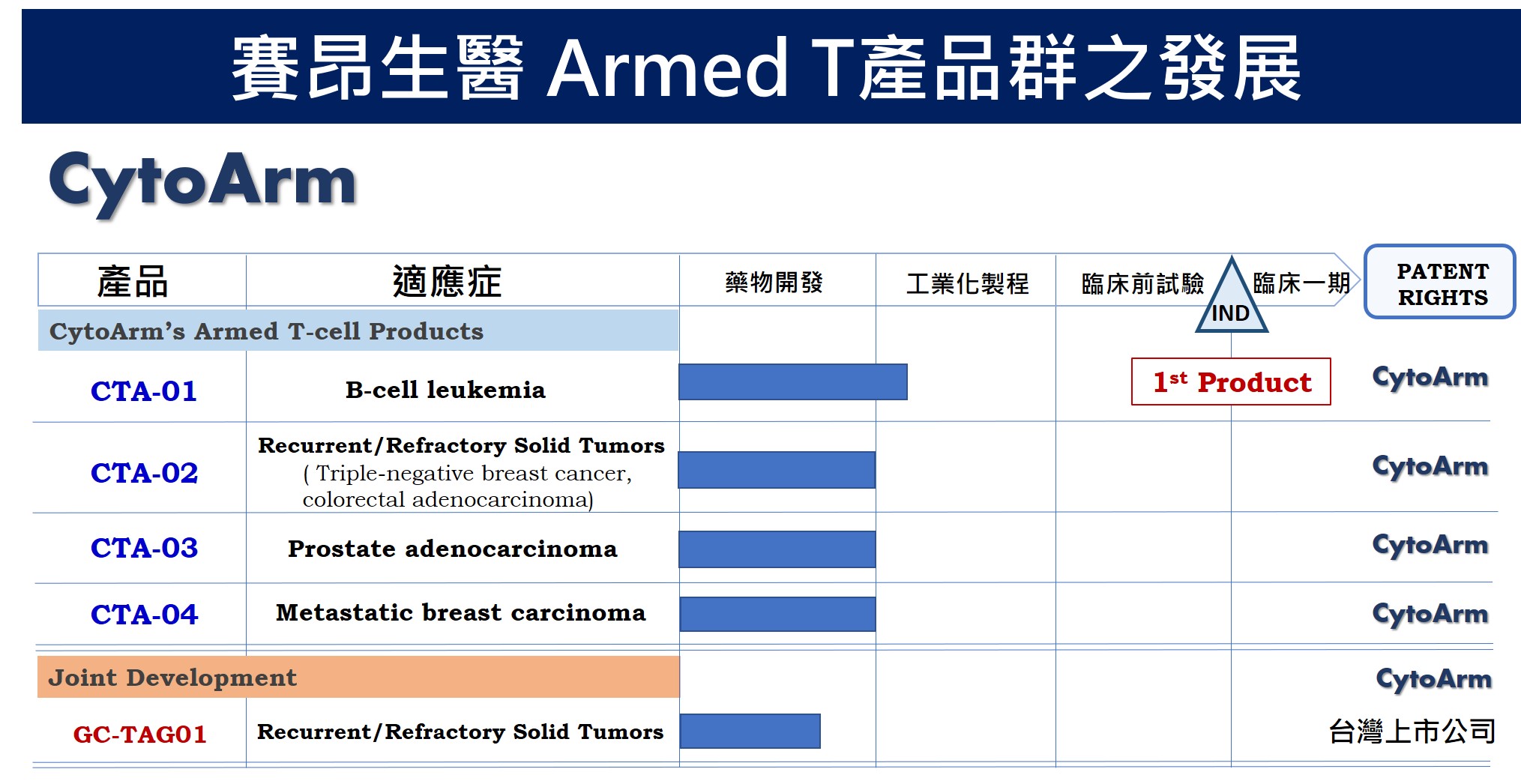
| 產業應用性 | 武裝型T細胞療法專利不受限於當前CAR-T細胞療法專利,能在全球T細胞療法市場搶占一席之地。本技術平台之產業模式:1. 與藥廠共同開發新型anti-cancer/anti-CD3 BsAb,共享專利與技轉金;2.技轉特定武裝型T細胞及其適應症予國際藥廠;3. 商品化武裝型T細胞醫療產品,推動上市。 |
||
| 關鍵字 | 武裝型T細胞 雙功能抗體 (BsAb) 以雙功能抗體培育腫瘤專一性T細胞 癌症 免疫療法 T細胞療法 實體腫瘤 血癌 惡性轉移腫瘤 非病毒基因轉殖技術 | ||
| 備註 | 附件資料 |
||
- 聯絡人
- 陳挺宇
- 電子信箱
- d339104002@tmu.edu.tw
其他人也看了