| Summary |
In this study, we wish to enhance gene engineering efficiency in E. coli and cyanobacteria by using CRISPR and CRISPRi system and achieve the purpose of bio-chemical production. In the beginning, we have developed a methodology that exploits and combines CRISPR and CRISPRi for the genome engineering and metabolic engineering of E. coli and cyanobacteria. we final combined both CRISPR and CRISPRi for metabolic engineering in E. coli and cyanobacteria, which improved the 1,4-BDO production and succinate. |
| Scientific Breakthrough |
(1) CRISPR/Cas9 can trigger foreign gene integrate into the genome of E.coli and cyanobacteria.
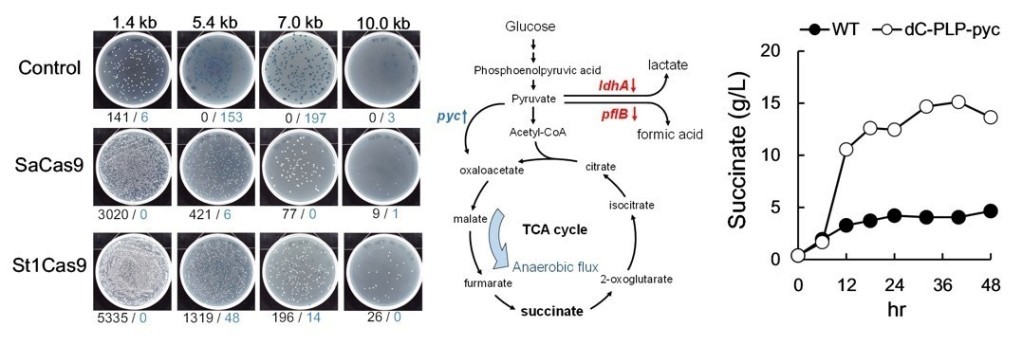
(2) By using CRISPR/Cas9 andλ-Red system in E.coli, we can integrate foreign DNA fragment (uo to 7kb). The recombinant efficiency can be enhance to 8-23 fold.
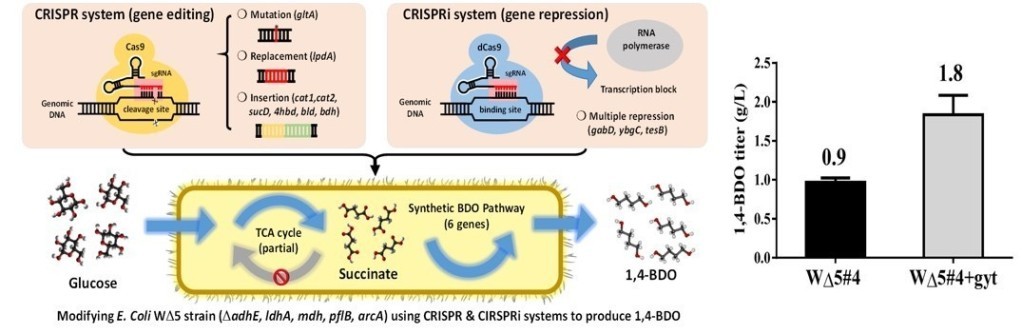
(3) By using CRISPR/Cas9 and CRISPRi, we regular the metabolic pathway of E. coli to produce 1,4-BDO. We replace the lpdA gene to foreign lpdA gene and point mutate the gltA gene. Then, the gene cat1, sucD, 4hbd, cat2, bld, bdh were be integrated into gene sad. Finally, we enhance the titer of 1,4-BDO from 0.9 g/L to 1.8 g/L(about 100% increasing)。
(4) In this study, we proved the sgRNA only work with their own Cas9 protein.
(5) The larger (from 7kb to 10 kb) fragment of foreign DNA can be integrated into the genome of E.coli by CRISPR/St1Cas9 system.
(6) Furthermore, we develop a metabolic tool which combine with CRISPR/CRISPRi and enhance the succinate production. The succinate production of WT E.coli is about 0.9 g/L. After we remove gene adhE and add gene pyc for increasing of succinate production in E. coli, the results showed succinate production enhanced about 167% (1.5g/L). The titer of succinate can be further increased to 178%(2.5g/L) if we decreased lactate and ethanol production and final succinate production reached 15 g/L in 48hr.
(7) In cyanobacteria, CRISPR/Cas9system can enhance the correct homologous recombination efficiency to 57%. Besides, we also successfully short the HR arm from 1000 bp to 400 bp by using CRISPR/Cas9 system.
(8) One challenge to genome engineering of PCC 7942 is the oligoploidy nature. Traditional homologous recombination method does not guarantee foreign gene integration into all chromosomes, thus requiring continuous streaking with increasing antibiotic concentration. Since CRISPR-Cas9 triggered effective DSB in all chromosomes and cell death, we hypothesized that CRISPR-Cas9 was able to enhance the chance of simultaneous gene integration into all chromosomes. As the results show we get stable recombinant strain and reduce the time of segregation.
(9) In this study, we proved CRISPRi system can regular gene expression and suppress target gene can up to 99%. The By using CRISPRi system, the gene can be suppressed very stable and didn’t cause any negative influence to cell.
(10) The succinate production increased about 12.5 fold (≈0.58-0.63 mg/L) by using CRISPRi system to interfere gene glgC and sdhB. |