| Technical Name | Acute Low dose Ketamine intravenous (IV) infusion for treatment resistant depression& suicidal cases | ||
|---|---|---|---|
| Project Operator | Cheng Hsin General Hospital | ||
| Project Host | 蘇東平 | ||
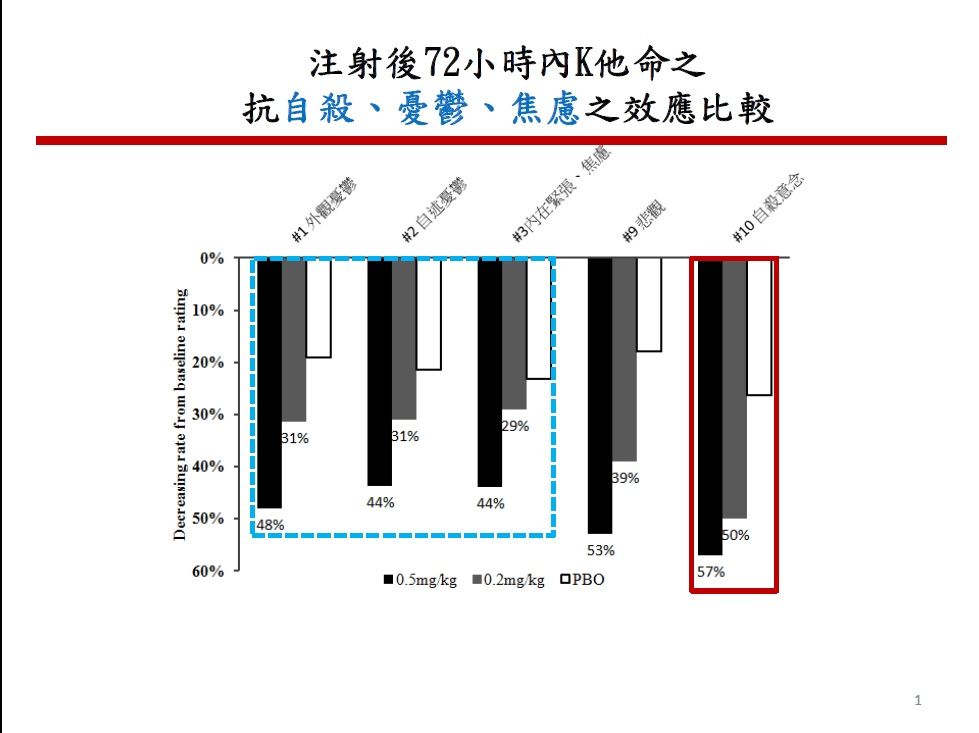
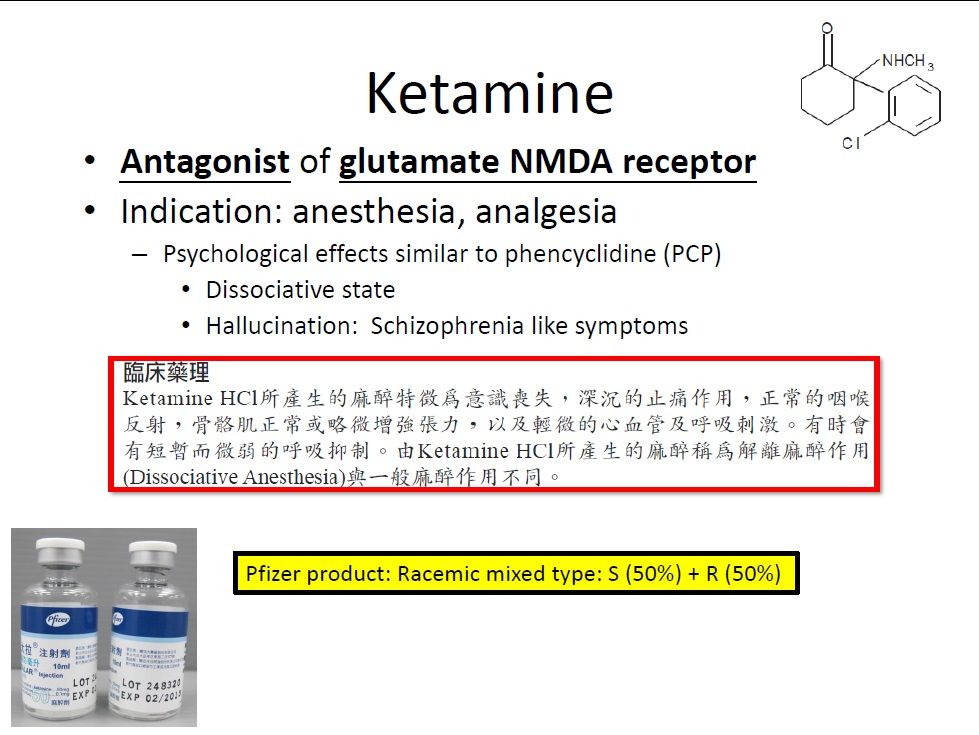
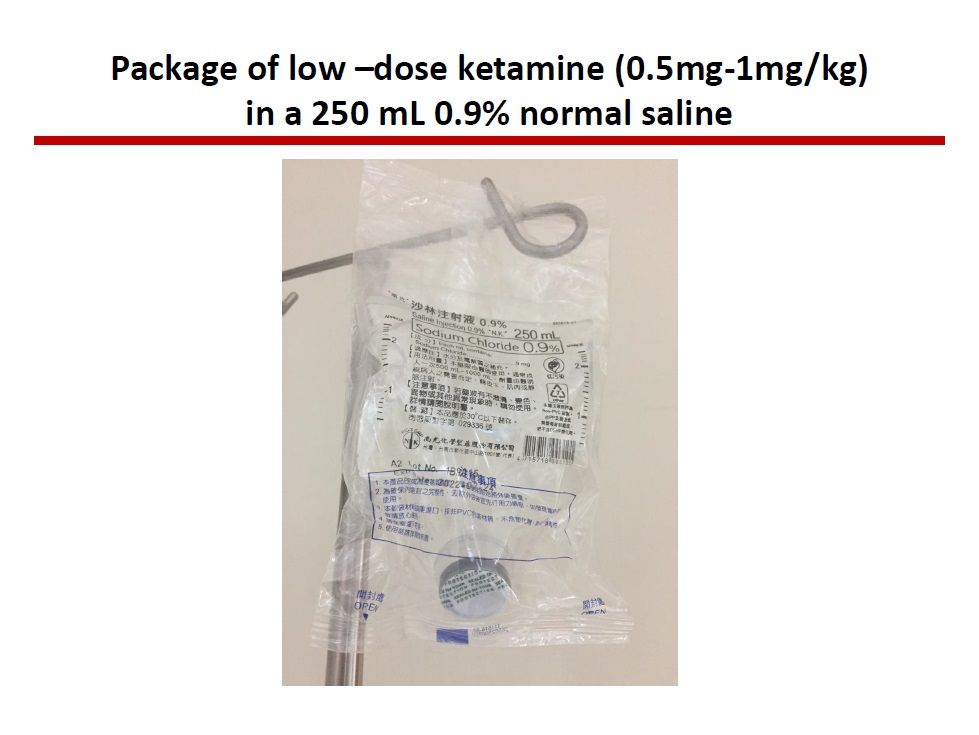
| Summary | We propose a new dosage form with 0.5mg-1.0mg/kg, mixed with 0.9% normal saline solution 250 ml, (i.e., 32.5-65 mg / 250 ml of 0.9% normal saline, according to the averaged body weight 65 kg). The advantage is convenient for use in acute emergency need. After this new product approved by TFDA, these bags are easy to distribute to anywhere needs. Ketamine is the class III controlled drug, this new product needs to be manufactured at the pharmaceutical company approved by TFDA. |
||
| Scientific Breakthrough | Breakthrough is that the new dosage form is ready for use, avoiding complicate procedures calculating the amount of ketamine, withdrawing ketamine, mixing with 250 ml 0.9% normal saline. These procedures is easy to make errors. Once the new dosage form got approval from TFDA, It will be easy and convenient to use this drug while indicated. It will not prohibit the physician, who is unfamiliar to this drug, to use. |
||
| Industrial Applicability | Indication are treatment resistant depression and suicidal ideation/attempt. Increased population of TRD and suicidal rate in Taiwan would urgently increase the needs of this new dosage form of ketamine. Not only the productivity but also the social economics be much increased. This new product should be conducted in the hospital requiring the presence of psychiatrists. Guideline for use will follow Esketamine(Janssen co.) proved by US FDA. |
||
| Keyword | New Dosage form product low dose ketamine Normal saline mixture treatment resistant depression suicidal ideation/ attempt depression low dose ketamine Normal saline New Dosage form | ||
- tomsu0402@gmail.com
other people also saw