| Technical Name | Development of Innovative Nanoparticles-lipoprotein to deliver CRISPR/Cas9 as gene therapy platform in ophthalmological inherited disease | ||
|---|---|---|---|
| Project Operator | National Yang-Ming University | ||
| Project Host | 邱士華 | ||
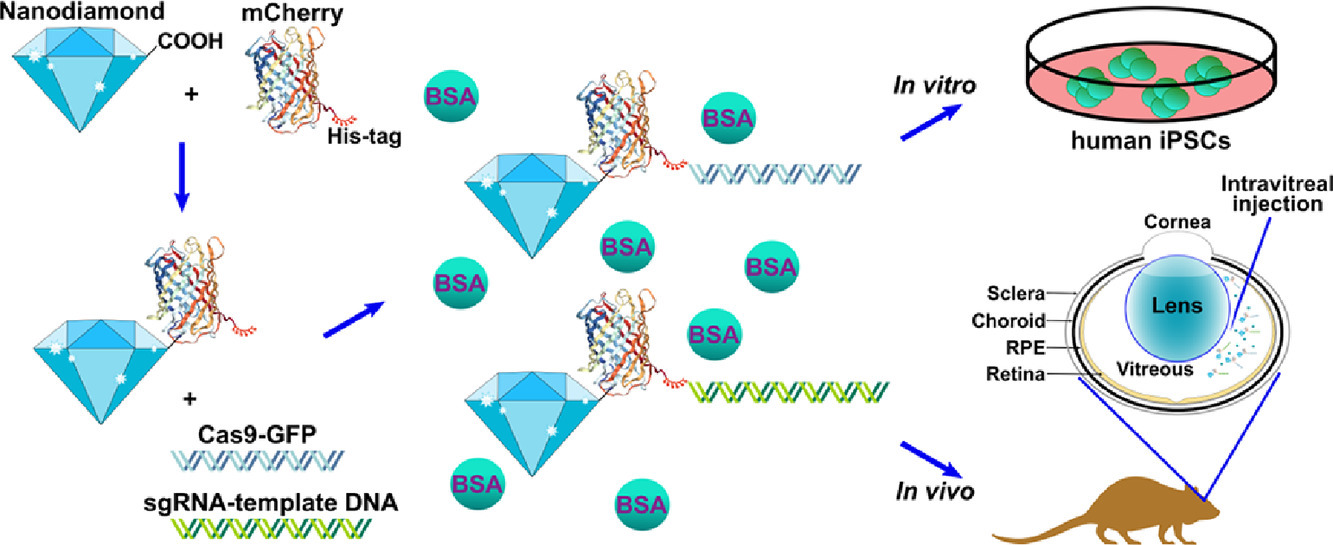
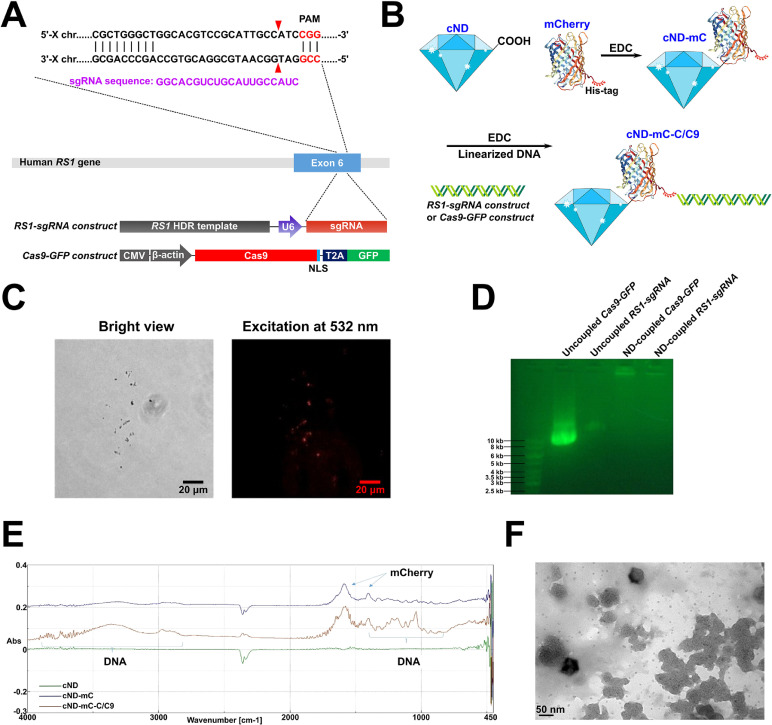
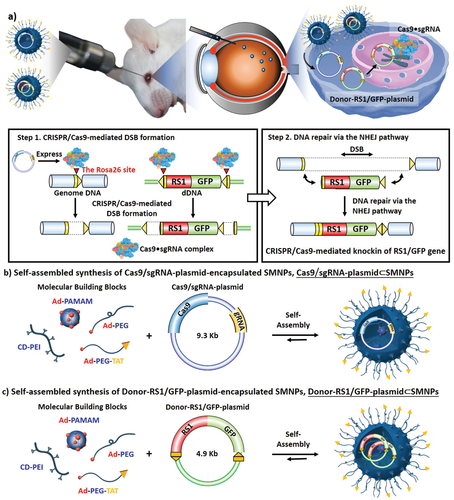
| Summary | The homology-independent targeted integration (HITI) strategy enables effective CRISPR/Cas9-mediated knock-in of therapeutic genes in nondividing cells in vivo, promising general therapeutic solutions for treating genetic diseases like X-linked juvenile retinoschisis. Herein, supramolecular nanoparticle (SMNP) vectors are used for codelivery of two DNA plasmids—CRISPR-Cas9 genome-editing system and a therapeutic gene, Retinoschisin 1 (RS1)—enabling clustered regularly interspaced short palindromic repeats (CRISPR)-associated protein 9 (CRISPR/Cas9) knockin of the RS1 gene with HITI. These SMNP vectors are then employed for CRISPR/Cas9 knockin of RS1/GFP genes into the mouse Rosa26 safe-harbor site in vitro and in vivo. Mice ocular organs retain their anatomical integrity, a single-copy 3.0-kb RS1/GFP gene is precisely integrated into the Rosa26 site in the retinas, and the integrated RS1/GFP gene is expressed in the retinas, demonstrating CRISPR/Cas9 knockin of RS1/GFP gene |
||
| Scientific Breakthrough | X-linked retinoschisis (XLRS) is a prevalent hereditary retinal disease, caused by mutations in RS1 gene, whose product is important for structural organization of the retina. The recent development of genome editing techniques such as CRISPR-Cas9 significantly improved the prospects for better understanding the pathology and development of treatment for this disease. Firstly, gene editing can allow development of appropriate in vitro and in vivo disease models; secondly, CRISPR-Cas9 can be applied for gene therapy by removing the disease-causative mutation in vivo. The major prerequisite for these approaches is to develop safe and efficient CRISPR-Cas9 delivery system. We were able to introduce Rs1 mutation into the mouse retina and, importantly, observed several XLRS-specific effects. |
||
| Industrial Applicability | Our technology is curablehighly bio-safecan be standardizedproduced in large quantities. Gene therapy will have a breakthrough impact. Effective therapeutic drugs will be developed for hereditary diseases,iPSC will be used as a testing platform for different diseases. In addition, cooperation will be carried out to enhance the technology of gene editing therapy. |
||
| Keyword | Nanodiamond X-linked juvenile retinoschisis, XLRS Retinoschisin, RS1 human induced pluripotent stem cells (hiPSCs) gene editing CRISPR-Cas9 inherited retinal diseases mouse retina HITI | ||
- s19609005@gm.ym.edu.tw
other people also saw